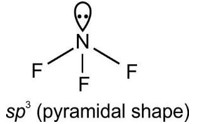
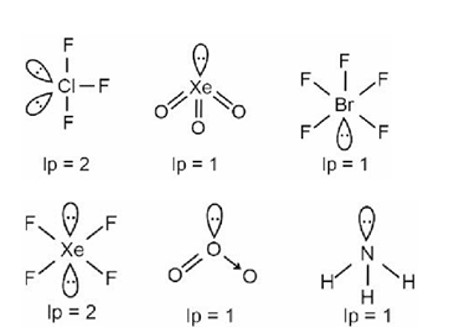
VSEPR Theory
Get insights from 17 questions on VSEPR Theory, answered by students, alumni, and experts. You may also ask and answer any question you like about VSEPR Theory
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
PF5, PCl5, PBr5, Fe (CO)5 Þ Trigonal bipyramidal
BrF5 Þ Square pyramidal
[PtCl4]2– Þ Square planar
SF6 Þ Octahedral
New answer posted
3 months agoContributor-Level 10
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
New answer posted
3 months agoContributor-Level 10
Total number of electron in Ti = 22
Total number of electron in Ti? = 22 – 4 = 18 So EAN value of Ti = 18 + 12 + 4 = 34
New answer posted
4 months agoContributor-Level 9
Water has only two lone pair and XeF4 has two lone pair electron in opposite plane of the central atom.
New answer posted
4 months agoContributor-Level 10
A central atom having two lone pairs and three bond pairs reflects sp³d hybridization and a corresponding T-shaped geometry.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers