Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
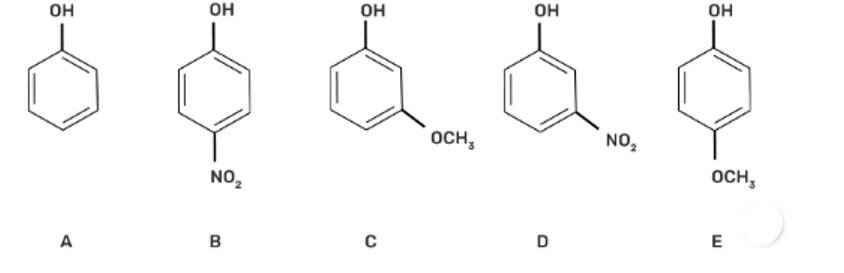
(II) b > d > a > c > e
The acidic strength decreases with increasing stability of the conjugate base of the given alcohol. -NO2 group at the para position in compound (b) is the most acidic due to the -M effect of the -NO2 group.
Compound (d) has -I effect on the conjugate base, whereas the -OCH3 group has + M effect on the conjugate base and hence decreases the acidity of compounds (c) and (e).
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(IV) m-Chlorophenol
Phenols are more acidic than sp3 and sp2 hybridized alcohol. The m-Chlorophenol is more acidic than phenol due to the -I effect (dominated by the + R effect) of chlorine atom increasing the acidity of phenol.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(IV) o-methoxy phenol
The stability of the conjugate base determines the acidic character of the compound. Higher the stability of the conjugate base, the higher is the acidic character.
Phenol, O-nitrophenol, O-methyl phenol, and O-methoxy phenol all are aromatic compounds. In aromatic compounds, the negative charge of the conjugate base charge is delocalised. The delocalisation of negative charge gives additional stability to the compound.
Ethanol is not aromatic and it cannot delocalise the negative charge formed after the H+ ion leaves the c. So,
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
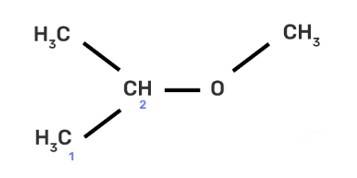
(II) 2-methoxy-2-methylethane
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure of the given compound is shown below:
The given compound is an alkane so suffix in our case will be –ane. An ether functional group is also present so prefix will be Alkoxy-.
To determine the name of a compound, identify the longest and continuous chain of carbon containing the functional group and count the number of carbon atoms in the chain as shown i
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
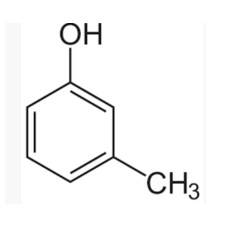
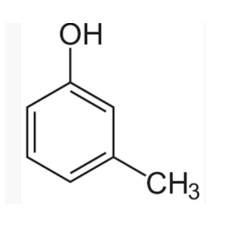
(I) 3-methylphenol
The m-cresol is an organic compound which contains a benzene ring, a -OH group and a methyl group. It has a methyl group substituted to meta position in the phenol ring. According to the IUPAC system, the -OH group is given more priority than the methyl group.
The cresols are also called hydroxytoluene or the methylphenols. These contain a benzene ring with one methyl and one phenol group substituted. There are three forms of cresols which are o-cresol, m-cresol and p-cresol. m- cresol is a colourless liquid and is viscous in nature.
In case of m-cre
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(III) 5-Chlorohexan-2-ol
The IUPAC naming of the given compound are as follows:
Word root: It depends upon the number of carbon atoms in the longest continuous carbon chain selected, called the parent chain. Depending upon the number of carbons in the chain the compound is assigned a word. The given compound contain 6 carbon attached in the continuous the longest chain and word root for 6 carbon atoms is 'hex'.
The suffix- A suffix is added after the word root to indicate the nature of the carbon-carbon bond. The given carbon chain contains a single bond then a s
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
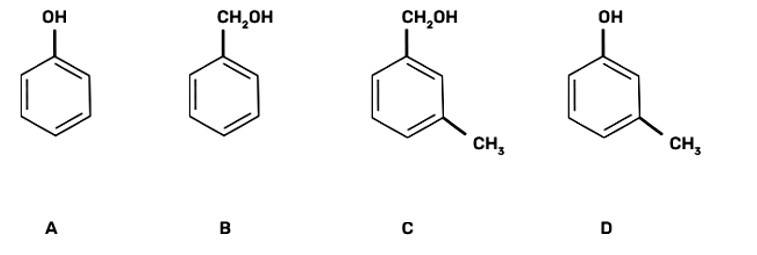
(III) B, C
The aromatic alcohols or aryl-alcohols are a class of chemical compounds containing a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group.
In the given question, the aromatic alcohols are those compounds in which the hydroxyl group is not directly attached to the benzene ring but is linked to a carbon atom situated in a side-chain. In short, we will search for that figure in which the hydroxyl group is linked to sp3 hybridised carbon.
So, compound B and C have -OH groups attached to sp3 hybridized -CH3 groups and hence are ar
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(II) Substitution reaction
Alkyl halides undergo substitution reactions to form corresponding alcohol.The halide ion, X- is substituted by OH- ion to form alcohol by nucleophilic substitution reaction.
R-X → R-OH
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(III) Treatment with pyridinium chlorochromate
The pyridinium chlorochromate is a complex of chromium trioxide with pyridine. This reagent is preferred for mild oxidation to form aldehydes and hence the oxidation to carboxylic acid is prevented.
Primary alcohols are oxidized to form aldehyde, whereas secondary and tertiary alcohols are oxidized to form ketones. The conversion of a hydroxyl group to aldehyde can be done by the removal of hydrogen atoms (Oxidation), looking at the following options; pyridinium chlorochromate and potassium dichromate are both oxidizing a
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers