Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
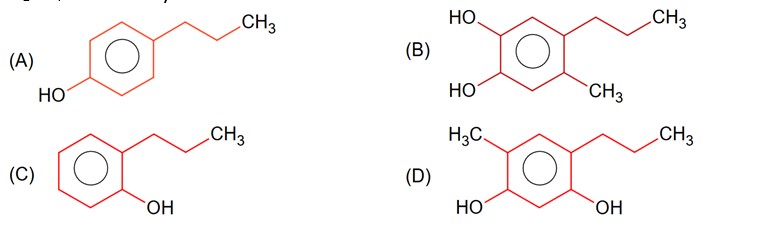
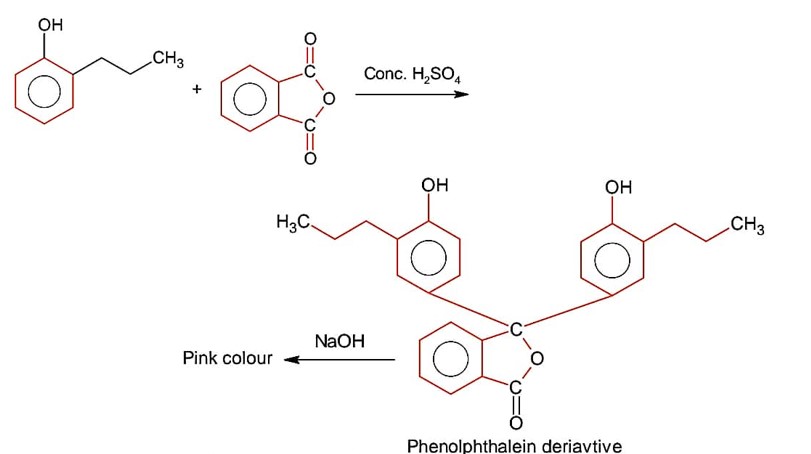
First reaction is EAS which will be given by that phenol derivative whose p-position is free for EAS reaction.
New answer posted
4 months agoContributor-Level 10
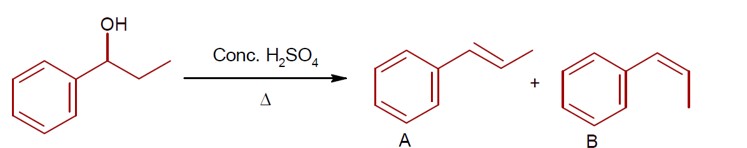
Trans product is more stable than Cis. ( Trans product feel less repulsion due to opposite direction)
New answer posted
4 months agoContributor-Level 10
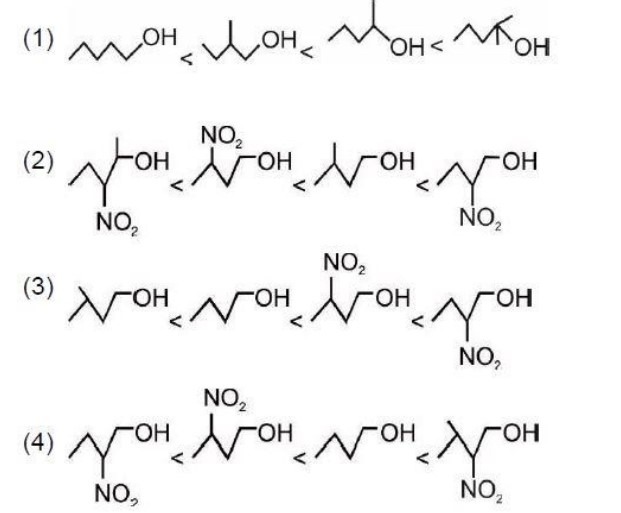
Formation of Conjugated diene in option 3 make the given reactant most reactive towards dehydration in acidic conditions.
New answer posted
4 months agoContributor-Level 10
Fe? O? + CO → 2FeO + CO?
The above reaction takes place at 500 – 800 K in blast furnace.
New answer posted
4 months agoContributor-Level 10
Pumice stone is an example of solid sol. In this type of colloid, the dispersion medium is solid and the dispersion phase is gas.
New answer posted
4 months agoContributor-Level 10
H? O < H? S < H? Se < H? Te
Down the group acidic strength increases
So pK? value decreases
New answer posted
4 months agoContributor-Level 10
R: CH? OH
Certain mild reducing agents like hypophosphorus acid or ethanol reduce diazonium salts to arene and themselves get oxidised to phosphorous acid and ethanal respectively.
New answer posted
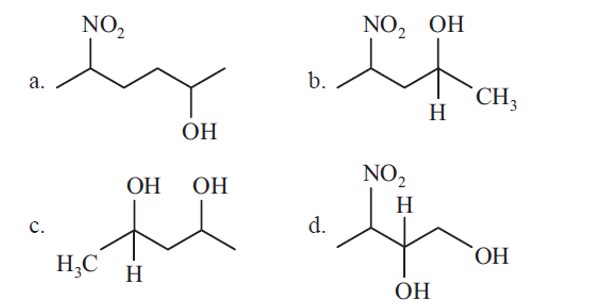
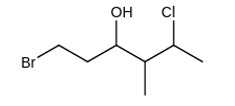
4 months agoContributor-Level 10
The IUPAC name giving the lowest number to the functional group obeying the lowest set of locants is 1-bromo-5-chloro-4-methylhexan-3-ol.
New answer posted
4 months agoContributor-Level 10
Statement I is true but statement II is incorrect as 3°-alcohols are the most reactive and give immediate turbidity with Luca's reagent.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers