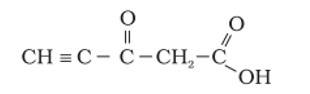Chemical Bonding and Molecular Structure
Get insights from 189 questions on Chemical Bonding and Molecular Structure, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Bonding and Molecular Structure
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The hybridization of Carbon 1 is sp, carbon 2 is sp, carbon 3 sp2, carbon 4 is sp3 and carbon 5 is sp2. The triple bond has 2 pie bonds and one sigma bond. Each double bond has one sigma and one pie bond. Every single bond is a sigma bond. Thus, the total number of sigma bonds is 11 and pie bonds are 4.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
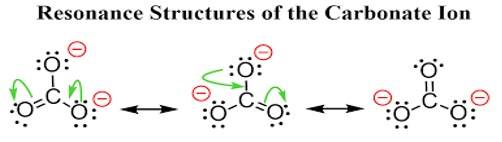
Ans: The carbonate ion CO32- can be best represented by its resonating structures. The carbonate ion cannot be represented by a single Lewis structure because the three carbon oxygen bond lengths are the same. This cannot be shown by a single Lewis structure. For showing the similar lengths of all the carbon to oxygen bonds three hybrid structures are constructed which are in resonance with each other.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
N—H, F—H, C—H and O—H
Ans: The ionic character in a molecular species is decided by the electronegativity difference between the two bonded atoms. Greater the electronegativity difference between two bonded pairs, the greater will be the ionic character. The electronegativity difference of the given species is
C - F = (2.5 - 2.1) = 0.4
N -H = (3.0 - 2.1) = 0.9
O - H = (3.5 - 2.1) = 1.4
F - H = (4.0 - 2.1) = 1.9
According to the above given difference in the electronegativities, the order of increasing ionic character is:
C – H< N - H
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Ionic bonds are such bonds in which complete transfer of electrons from one atom to another atom. Due to complete transfer positive and negative ions are formed in this bond. The ions in this bond are held together by electrostatic force of attraction. The formation of calcium fluoride CaF2 leads to the formation of an ionic bond.
Ca→ Ca2+ + e - The electronic configuration of Ca= [Ar] 4s2 and Ca2+= [Ar]
F +e -→F- The electronic configuration of F= [He] 2p5 and F = [He ]2p6
Thus Ca2+ +2F- → CaF2
A covalent bond is forme
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i) Due to the electrostatic forces between the two opposite charges, ionic bonds are non-directional. The bonding direction does not matter as the electrostatic field of an ion is non-directional. Whereas, the formation of covalent bonds happens with the overlap of the atomic orbitals. The direction of the bonds is given by the direction of overlapping.
(ii) The hybridization of the oxygen atom in water molecules is 3 sp due to the presence of two lone pairs of electrons on the oxygen atom. Tetrahedral geometry is acquired by these four 3 sp hybridized or
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i) N2 → N2+ + e-
The electronic configuration of N2 is:
σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x = π2p2y σ2p2z
So, its bond order will be 3
The electronic configuration of N2+ is:
σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x = π2p2y σ2p1z
Its bond order will be 2.5
Hence the bond order decreases in this reaction N2 → N2+ + e-
(ii) O2→ O2+ + e-
The electronic configuration of O2 is:
σ1s2 σ∗1s2 σ2s2 σ∗2s2 s2pz2 π2p2y π2p2x π*2p1y π*2p1x
Its bond order will be 2
The electronic configuration of O2+ will be:
σ1s2 σ∗1s2 σ2s2 σ∗2s2 s2pz2, π2p2y , π2p2x, π*2p1x
Its bond
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The general sequence of the energy level of the molecular orbital is σ1s < *1s < 2s< *2s < 2px = 2py < 2pz
N2 = σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x = π2p2y σ2p2z
N2+ = σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x = π2p2y σ2p1z
N2- = σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x= π2p2y σ2p2z σ2p2x
N22+ = σ1s2 σ*1s2 σ2s2 σ*2s2 π2p2x = π2p2y
The formula for finding the bond order (B. O) for any molecular species is:
Bond order = ( Nb- Na)
Hence, the bond orders of the given molecular species are:
N2= ( 10-4)= 3
N2+= ( 9-4)= 2.5
N2-=
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The Lewis structures of the compounds are:
Lewis structure for nitric acid:
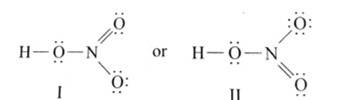
Lewis structure of nitrogen dioxide:
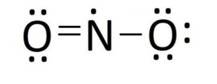
Lewis structure of sulphuric acid:
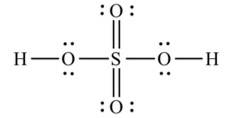
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Although the hybridisation of the central atom oxygen in both the molecules is 3 sp but dimethyl ether will have a higher bond angle than water molecule.
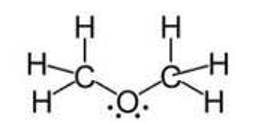
Due to the presence of two bulky methyl groups in dimethyl ether, the repulsive forces will be greater in them than the two hydrogens in water molecules. In dimethyl ether the -CH3 is a group attached to three hydrogen atom through s bonds. Thus, the C- H bond pairs increase the electron density on the carbon atom which results in lone pair-bond pair repulsions. Due to this lone pair-bond pair repulsions, the bon
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In PCl5, P has 5 valence electrons in orbitals and make 5 bonds with 5 Cl atoms, it will share one of its electrons from 3s to 3d orbital, therefore the hybridization will be sp3d and the geometry will be trigonal bipyramidal. IF5, the Iodine atom has 7 valence electrons in molecular orbitals it will form 5 bonds with 5 Cl atoms using 5 electrons from its molecular orbital, two electrons will form one lone pair on Iodine atom, which gives the square pyramidal geometry.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers