Chemical Bonding and Molecular Structure
Get insights from 189 questions on Chemical Bonding and Molecular Structure, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Bonding and Molecular Structure
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Hybridisation in the case of PCl5 :
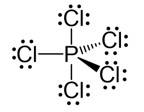
The hybridisation of phosphorus PCl5 is sp3d and it has a trigonal bipyramidal geometry. The axial bonds in PCl5 are slightly longer as compared to the equatorial bonds because axial bonds experience greater repulsive forces from other bonds as compared to the equatorial bonds.
Hybridisation in the case of SF6:
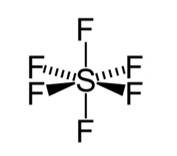
The hybridisation of sulphur in SF6 is sp3d2 and the molecule has an octahedral geometry. The bond length of axial as well as the equatorial bonds are similar because all the bonds in octahedral geometry experience similar r
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Formation of N2 molecule: Electronic Configuration
σ1s2 σ* < 1s2 < 2s2 < *2s2 < [ 2p2x = π2p2y] < 2p2z
The bond order will be:
( 10-4)= 3.0
Bond order indicates the number of bonds in a diatomic molecule. Triple bond N=N.
Formation of F2 molecule: Electronic Configuration
σ1s2 < *1s2< 2s2 < *2s2 < 2p2z < [ 2p2x = π2p2y] < [ *2p2x = π*2p2y] σ*2p2z
The bond order will be:
( 10-10)= 0
Hence there will be no bond formation in between the neon atoms.
New question posted
6 months agoNew answer posted
7 months agoContributor-Level 10
4.60. Lattice energy is defined as the energy released when one mole of crystalline solid is formed by the combination of oppositely charged ions.
(i) As the magnitude of charge on an ion increases there will be greater force of interionic attraction and hence greater will be the value of Lattice energy,
(ii) Smaller the. size of the ions> lower will be the internuclear distance and thus greater will be the Lattice energy,
New answer posted
7 months agoContributor-Level 10
4.59. The outer shell electrons are shown as dots surrounding the symbol of the atom. These symbols are known as Lewis symbols or Lewis structures.

New answer posted
7 months agoContributor-Level 10
4.58. (i) The molecule should contain highly electronegative atom like hydrogen atom.
(ii) The size of electronegative atom should be small.
New answer posted
7 months agoContributor-Level 10
4.57. Bonding molecular orbital has lower energy and higher stability.
New answer posted
7 months agoContributor-Level 10
4.55. N2O is more polar than CO2.
This is because CO2 is linear and symmetrical. Its net dipole moment is zero.
N2O is linear but not symmetrical.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
