Chemical Bonding and Molecular Structure
Get insights from 189 questions on Chemical Bonding and Molecular Structure, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Bonding and Molecular Structure
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
The electronic configuration of A 1S2 2S2 2P6 is having no unpaired electrons in the outer most shells. So, an atom having no unpaired electron in its valence shell is said to be stable.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
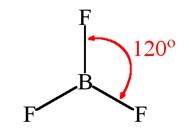
For a molecule having a hybridization of sp2, its geometry in plane is trigonal planar. For a molecule having a trigonal planar geometry, its bond angle will be 120°
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
The electrons involved in the formation of any chemical bond are the valence shell electrons. In the given electronic configuration,1s2 2s2 2p6 3s2 3d2 4s2 the valence shell electrons are in d-orbital and s-orbital. So, the four electrons involved in the chemical bond formation will be 3d2 4s2.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
Hydrogen bond is the strongest in that molecules have a higher difference in electronegativity. Due to the small size of the oxygen atom, it has the highest electronegative character. So, H2O molecules will have the strongest hydrogen bonding
HCl, HI and H2Sdo not have hydrogen bonding between their atoms. So, H2O will have the strongest hydrogen bonding.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii)
(i) The molecule XeF4 has a square planar geometry and the bond lengths of all the fluoride atoms attached to xenon atoms are equal.
(ii) The molecule BF4 - has a tetrahedral geometry and the bond lengths of all the fluoride bonds attached to boron are equal.
(iii) In the molecule C2H4 all the bonds are not equal. The bond length of the double bond between the carbon atoms is 134 pm and the bond length of CH- is 110 pm.
(iv) The molecule SiF4 has a tetrahedral geometry and the bond lengths of all the fluoride atoms attached to silicon atoms are equal.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii)
The molecular orbital diagram of O22-, π∗2Px and π∗2Py molecular orbitals are completely filled, which are partially filled in O2. These are no unpaired electrons. So, O22-does not contain unpaired electrons.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii)
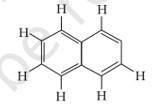
A double bond has one s bond and one π bond. In the above given structure 5π bonds are present. The s bonds are the additions of all the single bonds in the structure. There are 19s bonds in the above given structure.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
(i) The molecule of BH4 - has four bond pairs and zero lone pair of electrons, so it will be a tetrahedral molecule.
(ii) NH2 - has two bond pairs and two lone pairs of electrons on the nitrogen atom. So, it will have a bent geometry.
(iii) CO3 2- has three bond pairs and no lone pairs of electrons on carbon atoms. So, it will have a trigonal planar geometry.
(iv) H3O+ has three bond pairs and one lone pair of electrons on oxygen atoms. So, it has a pyramidal geometry.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
The number of bond pairs on the nitrogen atom of NO3- are 4 and the structure of - NO3 does not have any lone pairs of electrons. The structure of NO3- molecule is:
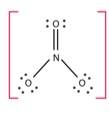
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
In a polyatomic molecule or an ion, the formal charge of an atom is defined as the difference between the valence electrons present in that atom and the number of electrons assigned to that atom in the Lewis structure.
Formal charge = No. of valence electrons - (No. of lone pair
Formal Charge = 6 -
Formal Charge = -1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers