Chemical Kinetics
Get insights from 144 questions on Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
4.12 2NH3 (g)? N2 (g) + 3H2 (g)
Rate of zero order reaction is equal to rate constant. i.e. Rate = 2.5 * 10-4mol L-1sec-1.
According to rate law,
-d [NH3] / 2dt = d [N2] / dt
2.5 * 10-4mol L-1sec-1 = d [N2] / dt
i.e. the rate of production of N2 is 2.5 * 10-4mol L-1 sec-1.
According to rate law,
-d [NH3] / 2dt = d [H2] / 3dt
d [H2] / dt = -3 X d [NH3] / 2dt
i.e. rate of formation of H2 is 3 times rate of reaction = 3 * 2.5 * 10-4mol L-1sec-1
= 7.5 * 10-4mol L-1sec-1
Rate of formation of N2 and H2 is 2.5 * 10-4 mol L-1sec-1 and 7.5 * 10-4 mol L-1sec-1 respectively
New answer posted
8 months agoContributor-Level 10
4.11 (a) Rate = k [A] [B]2, Rate = 0 * 10-6 [0.1] [0.2]2
Rate = 8 * 10-9 mol L-1sec-1
(b) 2A +B = A2B
=0.06+ 0.18 = 0.02
Situation when A remains 0.06 mol L-1
Now, According to rate law, Rate = k [A] [B]2
Rate = 2 * 10-6 [0.06] [0.18]2
i.e. Rate = 3.888 * 10-9 mol L-1sec-1
Initial rate of reaction is 8 * 10-9 mol L-1sec-1. and rate when concentration of A is 0.06 mol L-1 is 3.888 * 10-9 mol L-1sec-1.
New answer posted
8 months agoContributor-Level 10
4.10 (i) 3NO (g)? N2O (g)
Rate = k [NO]2
Here rate law is dependent on concentration of NO only. And power raised to concentration of NO is 2. Hence order of reaction is 2.
To find the dimensions of rate constant (K)- We know the rate of chemical reaction is measured in (concentration / time). So, the unit of K will be
(concentration) / (time) (concentration)2
i.e. [concentration]-1 [ time]-1 . If concentration is in mol L-1 and time is in sec. then dimensions of k will be : mol-1 L sec-1
(ii) H2O2 (aq) + 3I- (aq) + 2H+? 2 H2O (l) + I3-
Rate = k [H2O2] [I-]
Here rate law is dependent on concentration of H2O2 and I-. And power ra
New answer posted
8 months agoContributor-Level 10
4.9 Given-
Energy of activation, Ea= 209.5 kJ mol–1
Ea in joules = 209.5 * 1000 = 209500 J mol–1
Temperature, T = 581K
Gas constant, R = 8.314 J K-1 mol-1
Using the Arrhenius equation,
k = Ae-Ea/RT
In this equation, the term e-Ea/RT represents the number of molecules which have kinetic energy greater than the activation energy, Ea.
∴The number of molecules = e-Ea/RT → Equation 1. Substituting all the known values in equation 1, we get,
= e-209500/8.314X581
=e-43.3708
Finding the value of anti ln (43.3708), we get, 1.47 * 10-19
The fraction of molecules of reactants having energy equal to or greater than activation energy is 1.47 * 10-19
New answer posted
8 months agoContributor-Level 10
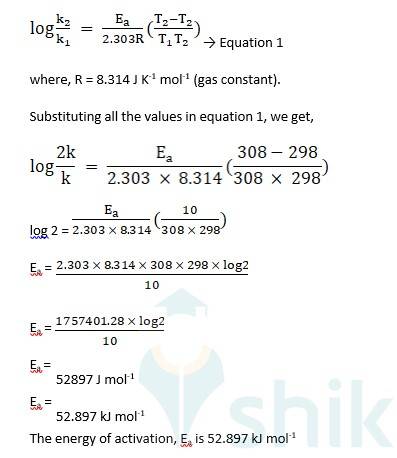
4.8 Given-
Initial temperature, T1 = 298 K
Final temperature, T2 = 298 K + 10 K = 308 K
Knowing that the rate constant of a chemical reaction normally increases with increase in temperature, we assume that,
Initial value of rate constant, k1 = k
Final value of rate constant, k2 = 2k
Using Arrhenius equation,
New answer posted
8 months agoContributor-Level 10
4.7 The rate constant of a chemical reaction normally increases with increase in temperature. It is observed that for a chemical reaction with rise in temperature by 10°C, the rate constant is nearly doubled and this temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation,
k = A
Thus, the rate constant of a chemical reaction normally increases with increase in temperature.
New answer posted
8 months agoContributor-Level 10
4.6 Given-
t1/2 = 60 mins
Using the formula for half life, t1/2 = 0.693/k, we get,
k= 0.693 / t1/2
k= 0.693 / 60
∴k = 1.155 * 10-2 min-1
The rate constant of the reaction, k is 1.155 * 10-2 min-1
New answer posted
8 months agoContributor-Level 10
4.5 Given-
Rate constant, k = 1.15 * 10-3 s-1
innitial quantity R0 = 5g
Final quantity, R = 3 g
According to the formula of first order reaction
K = 2.303/ t log R0 / R
1.15 X 10-3 = 2.303 /t log 5/3
t = 2.303 / 1.15 X 10-3 log 5/3
t = 443.8 s
t = 4.438 * 102 s
The time taken for 5g of the reactant to reduce to 3 g is 4.438 * 102 s
New answer posted
8 months agoContributor-Level 10
4.4 Let the reaction be X→Y
As this reaction follows second order kinetics, the rate of the reaction will be,
Rate = k (X)2 → Equation 1
Since the concentration of X is increased three times, Equation 1 will become,
Rate = k (3X)2
= k * 9 (X)2
∴ The rate of formation of Y will become 9 times.
Thus, the rate of formation of Y will become 9 times
New answer posted
8 months agoContributor-Level 10
4.3 The order of the reaction is sum of the powers of concentration of reactants in the rate law. According to this,
The order of the reaction = 1/2 + 2
= 2 1/2 or 2.5
The order of the reaction is 2.5
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers
