Chemical Kinetics
Get insights from 144 questions on Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
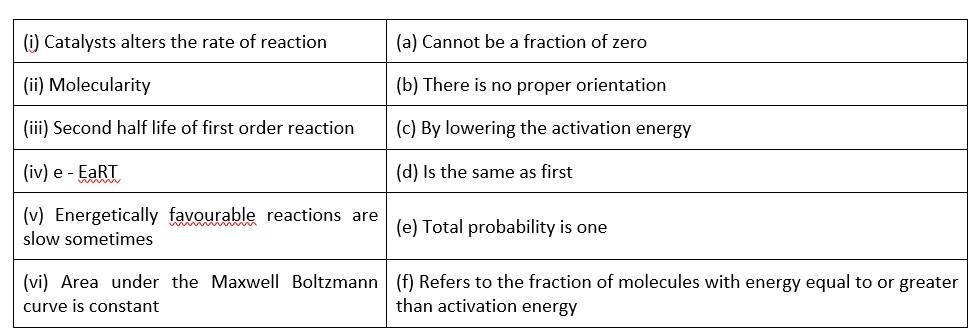
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (c) ; (ii)- (a) ; (iii)- (d); (iv)- (f); (v)- (b); (vi)- (b)
A catalyst can influence the rate of a process by lowering the activation energy
When it comes to molecularity, there can't be a fraction or a zero.
The second half life of a first order reaction is the same as the first.
Activation energy refers to the percentage of molecules with an energy equal to or greater than activation energy.
Correct orientation is not always present when it comes to energetically favourable activities.
Because the area under the Maxwell Boltzmann curve is constant, the total probability
New answer posted
7 months agoContributor-Level 10
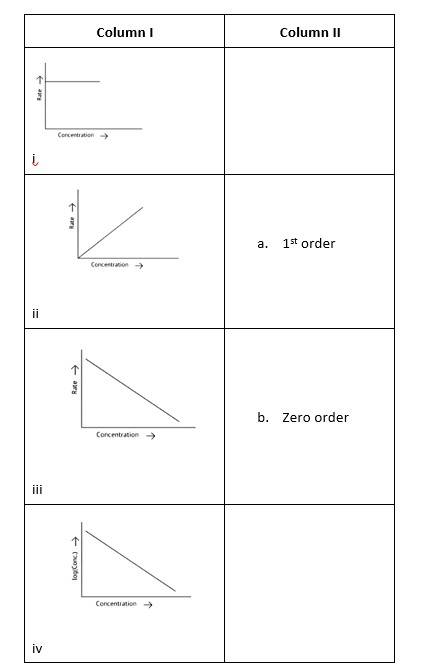
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (a) ; (ii)- (b) ; (iii)- (b); (iv)- (a)
A zero-order reaction is one in which the reactant concentrations do not change over time and the rate of concentration remains constant.
A first-order reaction is one in which the rate of the reaction is linearly proportional to the concentration of only one ingredient. In other terms, a first-order reaction is a chemical reaction whose rate is determined by changes in only one of the reactants' concentration.
New answer posted
7 months agoContributor-Level 10
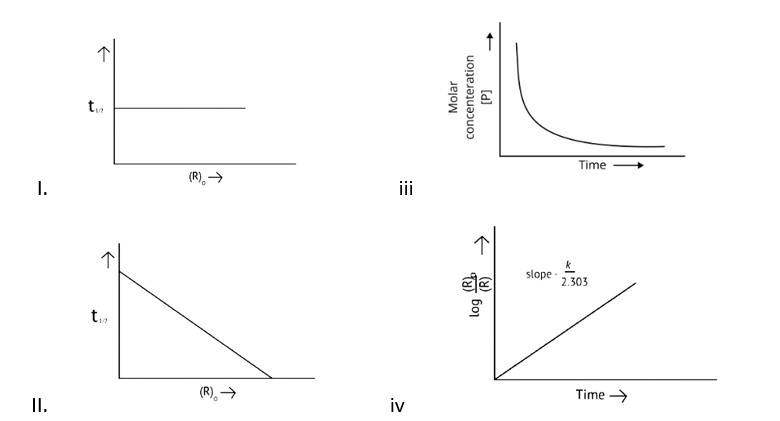
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
The pace of a reaction that is directly proportional to the concentration of the reacting substance is known as a first order reaction.

Y=mn
k = log
t = log
x= a-
x=
t = log
log2
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
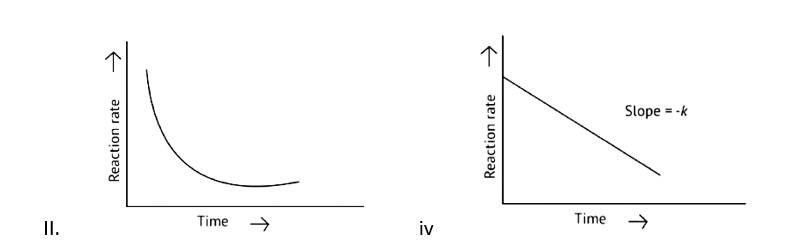
A Zero order reaction = [R] = ( - k)t + [R]0
y = (m * ) + c
x = t (time)
y = [R]concentration
Slope (m) = - k
Intercept (c) = [R]0
= - k
= - kto
Rate ∝ t0
New answer posted
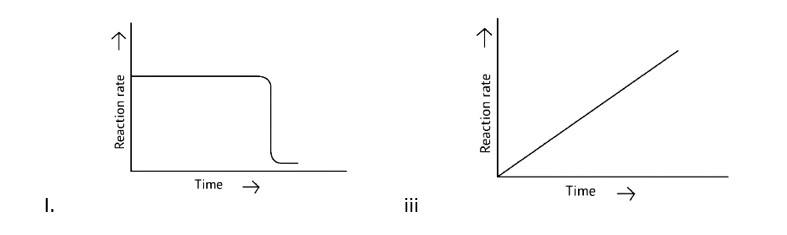
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: B, C and D
Catalysts reduce the activation energy of a process and give an alternate path by reducing or raising the activation energy between reactants and products, altering the reaction's enthalpy change. As a result, B, C, and D statements are the proper responses
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and B
Arrhenius equation k = Ae –
When the temperature rises, value falls and –
As a result, e- increases, and it is certain that k and T increases as well. As a result, Option A is proven.
Catalyst increases the rate of reaction by lowering the activation energy. Thus option B is also correct.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
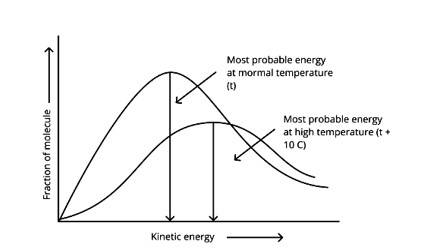
According to the graph, the area under the cure must not vary as the temperature rises.T2 > T1
The energy level of T2 is higher than T1.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and C
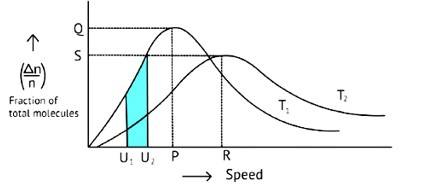
The Maxwell Boltzmann distribution, often known as the Gibbs distribution, is a measure that expresses the likelihood of a system being in each state as a function of the energy of that state.
Look at the graph.
T2 > T1
The fraction of molecules falls as the temperature rises because the area under the curve shrinks.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
Activation Energy is the amount of energy released when reactant molecules collide and form an activated complex. When energy is released, the complex decomposes into a product.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A, C and D
The pressure in this reaction is extremely high, and it becomes independent of the ammonia concentration. The metal surface becomes saturated with gas molecules when the rate of reaction=Rate constant. The rate of a zero-order reaction is independent of the concentration of reactants in the reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers