Chemical Kinetics
Get insights from 144 questions on Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Correct option: C
A catalyst allows a chemical reaction to occur at a faster rate or under different conditions than it would otherwise. As a result, a catalyst affects the reaction's enthalpy change, or heat. As a result, in the presence of the reaction, the enthalpy does not vary, i.e., it remains constant, and no heat is produced or absorbed.
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Correct option: B
The Arrhenius equation can be used to calculate the activation energy of a chemical process. At two temperatures, this determines the rate constants.
2.303log = =
New answer posted
7 months agoContributor-Level 10
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Option C
Gibbs energy of reaction - Gibbs free energy is a single-valued combination of entropy and enthalpy. The direction of a chemical reaction at constant temperature and pressure is predicted by Gibbs free energy.
Enthalpy of reaction – When one mole of matter is converted by a chemical reaction under specified conditions, the enthalpy of reaction is the change that occurs in the system.
Activation energy of reaction – The lowest amount of energy necessary to activate molecules to a state where they can perform physical and chemical transformations is known
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: C
The assertion is valid, but the reasoning is erroneous because Arrhenius equation rate constants are accurate for both simple and complex molecules with suitable orientation during effective collision and sufficient kinetic energy to cause chemical change.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: E
The claim is erroneous, but the reason is right, because the correct assertion is that effective collision leads to the production of a product, and the reason states that the conditions of collision theory must be met for the reaction to be completed.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: A
Both the assertion and the reason are valid, and the reason is a correct of the assertion since the enthalpy of reaction is the difference between the total enthalpy of the reactants and products in the presence of a catalys
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: E
The order and molecularity may or may not be the same, assertion is false, but reason is right. The sum of the power of the reactants is the order of reaction, which may be easily determined. The total of the stoichiometric coefficient of the rate-determining elementary step determines molecularity
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: B
Both assertion and reason are valid, but reason does not explain assertion because the order of reaction can be zero or even fractional because the order of reaction is proportional to the total of the power of the reactants.
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (a) ; (iii)- (d); (iv)- (c)
The rate rule was discovered experimentally and can be used to predict the reaction rate and reactant concentrations.
The rate constant is the proportionality constant that describes the relationship between the molar concentration of the reactants and the rate of a chemical reaction.
The rate constant (k) of a reaction is proportional to its temperature, i.e., for a given reaction at a given temperature, the rate constant (k) is constant.
The order of a reaction is a quantity that has been empirically determined. As a result
New answer posted
7 months agoContributor-Level 10
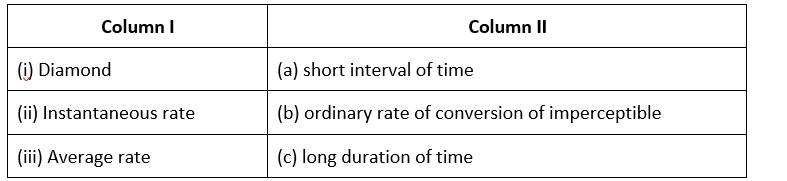
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (c) ; (iii)- (a)
The rate of conversion in diamond is normally imperceptible.
Long-term rate at a moment's notice
The average rate is only for a short time.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers