Chemistry Chemical Bonding and Molecular Structure
Get insights from 133 questions on Chemistry Chemical Bonding and Molecular Structure, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Chemical Bonding and Molecular Structure
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoBeginner-Level 5
The central atom of nitrogen has 5 valence electrons as per the electronic configuraion. During the formation of , 3 valence electrons forms three sigma bonds with hydrogen and one lone electron pair is left.
- The steric number of the ammonia molecule: SN=3 bonds +1 lone pair total electron domains.
- As per the steric number, there is hybridisation in ammonia.
- The lone pair causes repulsion, which leads to a trigonal pyramidal geometry with bond angles of .
New answer posted
5 months agoBeginner-Level 5
You can check the below given table for the comarative differences between valence bond theory and molecular orbital theory.
| Feature | Valence Bond Theory (VBT) | Molecular Orbital Theory (MOT) |
|---|---|---|
| Basic Concept | Overlap of atomic orbitals. | Atomic orbitals combine to form molecular orbitals |
| Bond Formation | Due to head-on (? ) or sideways (? ) overlap of atomic orbitals. | Linear combination of atomic orbitals (LCAO) to form bonding and antibonding MOs. |
| Explanation of Magnetic Behavior | Often fails to explain magnetism of molecules (e.g., O? is paramagnetic). | Accurately explains paramagnetism/diamagnetism (O? is paramagnetic due to unpaired electrons in antibonding orbitals). |
| Bond Order | Not clearly defined. | Bond order = ½ (No. of bonding electrons - No. of antibonding electrons) |
| Electron Delocalization | Electrons are localized between two atoms. | Electrons may be delocalized over multiple atoms. |
| Energy Consideration | Considers only overlapping orbitals and their energy. | Considers combination and energy differences of atomic orbitals. |
| Applicability | Works well for simple molecules like H? , HF, etc. | Better for explaining molecules like O? , N? , and ions like NO? , CN? |
New answer posted
5 months agoBeginner-Level 5
The reason why bond angle is larger in than are given below.
- In , there is one lone pair on the nitrogen atom increases repulsion, while the lone pair on phosphorus is in a higher energy orbital and causes less repulsion.
- Nitrogen is a small and electronegative atom whereas phosphorus is larger and less electronegative than nitrogen.
- In , the bonding orbitals are nearly pure p-orbitals, which are less directionals–p hybridization, on the other hand, the bond pairs remain fairly directed, leading to a larger bond angle.
Hence, the bond angle in is about 107° ( less than the ideal tetrahedral 109.5°) due to lone pair pres
New answer posted
5 months agoBeginner-Level 5
The NO (Nitric Oxide) has 11 valence electrons while the NO? (Nitrosonium ion) has 10 valence electrons due to removal of one antibonding electron. Due to this, the bond order of NO increases from? 2.5 to 3 in the NO? (Nitrosonium ion).
As per the NCERT, since the bond order of NO? (3) is higher than that of NO (2.5), the bond in Nitrosonium ion is stronger bond and the stronger the bond, the shorter bond length.
Hence bond length in NO? is shorter the NO.
New answer posted
5 months agoBeginner-Level 5
The difference in boiling or melting point, even after nearly the same molecular geometry, is because opf the presence of hydrogen bond. HF forms intermolecular hydrogen bonds due to fluorine's high electronegativity. This intermolecular hydrogen bonding requires more energy due to break the hydrogen bonds and melt or boil. So, stronger intermolecular forces of HF result in a higher boiling point.
New answer posted
5 months agoBeginner-Level 5
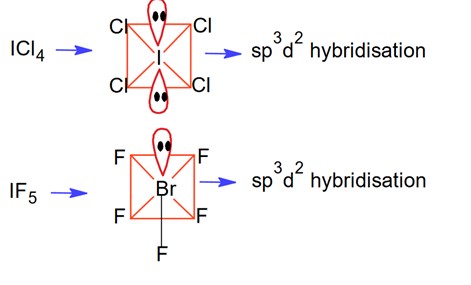
Xenon has 8 valence electrons. In , it forms two bonds with fluorine, leaving three lone pairs. The steric number =2 bonds +3 lone pairs .
As per the VSEPR Theory the electron? pair geometry due to SN = 5 will be trigonal bipyramidal. The hybridization type will be hybridisation. However, the three lone pairs occupy equatorial positions, resulting in a linear molecular geometry.
New answer posted
5 months agoContributor-Level 10
SO3 -> Sp2 Planar
BF3 -> Sp2 Planar
-> Sp2 Planar
SF4 -> Sp3d non-planar
H2O2 -> Sp3 Non-planar
PCl3 -> Sp3 Non –planar
[Al (OH)4]- -> Sp3 Non-planar
XeF4 -> Sp3d2 planar
XeO3 -> Sp3 Non-planar
-> Sp3 Non-planar
New answer posted
5 months agoContributor-Level 10
Higher melting point means more energy is required to break the ionic bond. Since the ionic bonds in MgO are stronger than in NaCl due to various reasons, It have higher melting and even boiling point. Reasons are listed below:
- In MgO, Magnesium and oxygen ions carry a +2 and -2 charge while sodium and in NaCl, chlorine ions carry a +1 and -1 charge. Due to higher charges more electrostatic attraction is involved.
- The smaller ionic radius of Mg2+ are smaller compared to Na+, makes the bond stronger in MgO.
- Due to larger elctrostatic force and smaller ionic radius, ionic bond in MgO are stronger, resulting in higher lattice energy.
- The stro
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers