Chemistry Electrochemistry
Get insights from 101 questions on Chemistry Electrochemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Electrochemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
E°_cell = E° (cathode)-E° (anode) = 0.16-0.52=-0.36V.
lnK = nFE°/RT = 1*0.36/0.025 = 14.4. Ans is x10? ¹, so 144.
New answer posted
5 months agoContributor-Level 9
Sol. E? cell = E? (Sn²? |Sn) - E? (Cu²? |Cu)
= -0.16 - 0.34 = -0.50V
ΔG? = -nFE? cell
= -2 * 96500 * (-0.5) = 96500 J
= 96.5 kJ = 96500 J
New answer posted
5 months agoContributor-Level 10
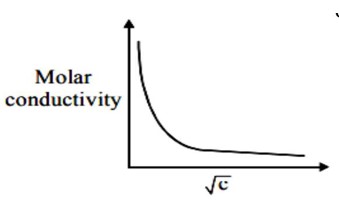
KNO? , HCl and NaCl are strong electrolytes for these electrolytes of Λ_m with √c will be liner which can be given as
Λ_m = Λ? _m - A√c for strong electrolyte
Since given variation is not linear it has to be a weak electrolyte
CH? COOH is a weak electrolyte
New answer posted
5 months agoContributor-Level 10
Each ion makes a definite contribution irrespective of the other ion
New answer posted
5 months agoContributor-Level 9
Fe? ³ + e? → Fe? ² E° = 0.77V
Zn (s) → Zn? ² + 2e? ; E° = 0.76V
Cell reaction: 2Fe? ³ + Zn → 2Fe? ² + Zn? ² E°cell = 1.53V
Ecell = E°cell - (0.059/2)log ( [Zn? ²] [Fe? ²]²/ [Fe? ³]²)
1.5 = 1.53 - (0.06/2)log (1 * [Fe? ²]²/ [Fe? ³]²)
-0.03 = -0.03 log ( [Fe? ²]/ [Fe? ³])²
1 = log ( [Fe? ²]/ [Fe? ³])² => [Fe? ²]/ [Fe? ³] = 10
Let total iron = T. [Fe? ³] + [Fe? ²] = T. [Fe? ³] + 10 [Fe? ³] = T. 11 [Fe? ³] = T.
fraction of Fe? ³ = [Fe? ³]/T = 1/11 ≈ 0.09
This solution seems to differ from the image. Let's follow the image's steps.
log ( [Fe? ²]/ [Fe? ³])² = 1 => ( [Fe? ²]/ [Fe? ³])² = 10
[Fe? ²]/ [Fe?
New answer posted
5 months agoContributor-Level 10
Λ? = K * 1000 / M = (2 * 10? * 10³) / 10? ³ = 20 Scm²mole? ¹
For weak acid (α) = Λ? / Λ? = 20 / 190 = 2/19
K? = Cα² / (1 - α) = 10? ³ * (2/19)² / (1 - 2/19)
= 10? ³ * (4/361) / (17/19) = 0.01238 * 10? ³
= 12.38 * 10?
So, ans is 12 (the nearest integer).
New answer posted
5 months agoContributor-Level 10
Main product of electrolysis of conc. H? SO? is H? S? O? i.e. HO? SO-OSO? H
New answer posted
5 months agoContributor-Level 10
Quantity of electricity required to reduce 1 mole of is 5F
So, for 5 mole 25F electricity is required.
New answer posted
5 months agoContributor-Level 10
Anode :
Cathode : 2Ag+(aq) + 2e- ® 2Ag(s)
Zn(s) + 2Ag+(aq) ® Zn2+ (aq) + 2Ag(s)
x = 147
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers