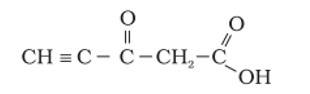Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Get insights from 99 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: All the C-Obonds in carbonate ion (CO32-) are equal in length due to the equivalent resonance of all the three carbon-oxygen bonds gets a double bond character atleast once. This type of double bond character happened throughout 3C- O skeleton, and hence all the bonds acquired equal bond length.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In compound BCl3, Boron has sp2-hybridisation and the shape is Triangular Planar.
In methane CH4, Carbon has sp3 -hybridization and shape are Tetrahedral.
In carbon dioxide CO2, carbon has sp-hybridisation and shape is Linear.
In ammonia NH3, nitrogen has sp3-hybridisation and shape is Pyramidal.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i)

(ii) 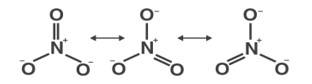
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i) According to the electronic configuration, a total of 8 electrons must be present in the valence shell of an element. Element X has 4 valence electrons, so it will share the remaining 4 electrons for the formation of the bond, the molecular formula will be XH4 . The element Y has 5 valence shell electrons, so it will form 3 bonds and the formula will be YH3 . The element Z has 7 valence shell electrons, so it will form one bond with hydrogen and has the molecular formula H-Z .
(ii) Elements X, Y and Z having, 5 and 4 7 valence electrons respectively belongs
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: BeCl2 has a linear structure
HOCl is also non-linear in structure.
H2O has a V-shaped structure.
Cl2O has a V-shaped structure.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The hybridization of Carbon 1 is sp, carbon 2 is sp, carbon 3 sp2, carbon 4 is sp3 and carbon 5 is sp2. The triple bond has 2 pie bonds and one sigma bond. Each double bond has one sigma and one pie bond. Every single bond is a sigma bond. Thus, the total number of sigma bonds is 11 and pie bonds are 4.
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The carbonate ion CO32- can be best represented by its resonating structures. The carbonate ion cannot be represented by a single Lewis structure because the three carbon oxygen bond lengths are the same. This cannot be shown by a single Lewis structure. For showing the similar lengths of all the carbon to oxygen bonds three hybrid structures are constructed which are in resonance with each other.
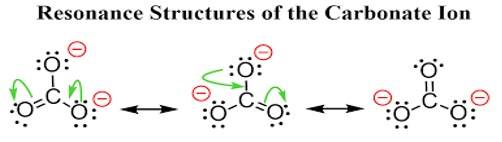
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
N—H, F—H, C—H and O—H
Ans: The ionic character in a molecular species is decided by the electronegativity difference between the two bonded atoms. Greater the electronegativity difference between two bonded pairs, the greater will be the ionic character. The electronegativity difference of the given species is
C - F = (2.5 - 2.1) = 0.4
N -H = (3.0 - 2.1) = 0.9
O - H = (3.5 - 2.1) = 1.4
F - H = (4.0 - 2.1) = 1.9
According to the above given difference in the electronegativities, the order of increasing ionic character is:
C – H< N - H
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Ionic bonds are such bonds in which complete transfer of electrons from one atom to another atom. Due to complete transfer positive and negative ions are formed in this bond. The ions in this bond are held together by electrostatic force of attraction. The formation of calcium fluoride CaF2 leads to the formation of an ionic bond.
Ca→ Ca2+ + e - The electronic configuration of Ca= [Ar] 4s2 and Ca2+= [Ar]
F +e -→F- The electronic configuration of F= [He] 2p5 and F = [He ]2p6
Thus Ca2+ +2F- → CaF2
A covalent bond is forme
New answer posted
8 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i) Due to the electrostatic forces between the two opposite charges, ionic bonds are non-directional. The bonding direction does not matter as the electrostatic field of an ion is non-directional. Whereas, the formation of covalent bonds happens with the overlap of the atomic orbitals. The direction of the bonds is given by the direction of overlapping.
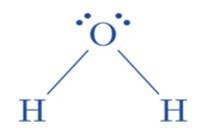
(ii) The hybridization of the oxygen atom in water molecules is 3 sp due to the presence of two lone pairs of electrons on the oxygen atom. Tetrahedral geometry is acquired by these four 3 sp hybridized or
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers