Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Get insights from 99 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
In molecular shape prediction, VSEPR (Valence Shell Electron Pair Repulsion) theory is important because based on the repulsions between the electron pairs around the central atom, it helps in predicting the three-dimensional shape of molecules. The 3D shape influences the molecule's chemical and physical properties including reactivity, polarity, and intermolecular forces.
New answer posted
8 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (iv)
Both the statements given in the assertion and reason are false.
The energy required to break the first O-H bond is not same than that of the second O-H bond in water molecule, that means the bond enthalpies of the two O-Hare different. This is because the electronic environment around the oxygen atom in water molecules is not the same even after the O-H bond breaks.
New answer posted
8 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (i)
The statements given in the assertion and reason, both are correct and the reason given is the correct explanation for the assertion. In NH3 only one lone pair of electron is present and due to that lone pair-bond pair repulsion occurs which forms a distorted tetrahedral geometry (pyramidal) .
In H2O two lone pairs of electrons are present on the oxygen atom and due to this lone pairlone pair repulsion occurs which shifts the bonds and forms a bent geometry. The lone pair-lone pair repulsion is more than the lone pair-bond pair repulsion. Thus, bond angle i
New answer posted
8 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Ans: Option (i)
Both the statements given in assertion and reason are correct and the reason given is the correct explanation for the assertion. The sodium chloride formed by the action of chlorine gas on the sodium metal has a complete octet. The valence shells of both the atoms are fulfilled, that is why attains an octet.
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Tetrahedral (c) sp3
(ii) Trigonal (a) sp2
(iii) Linear (b)sp
Explanation :-
(i) A tetrahedral molecule has four electron pairs and these make sigma bonds with each other. The s and p orbitals overlap with each other thus forming a sp3 hybridized molecule.
(ii) There are two possibilities for the central atom to have sp2 Either all the bonds are in place i.e, the bond pairs are all sigma bonds or pi bonds or there are only two bonds and one lone pair of electrons.
(iii) The sp hybridization involves the mixing of the val
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Hydrogen bond (d) HF
(ii) Resonance (e) O3
(iii) Ionic bond (b) LiF
(iv) Covalent solid (a) C
Explanation :-
(i) In hydrogen fluoride HF, hydrogen forms
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) NO (c) 2.5
(ii) CO (d) 3.0
(iii) O22- (a) 1.5
(iv) O2
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) H3O+ (e) Pyramidal
(ii) HC? CH (a) Linear
(iii) ClO2- &nb
New answer posted
8 months agoContributor-Level 10
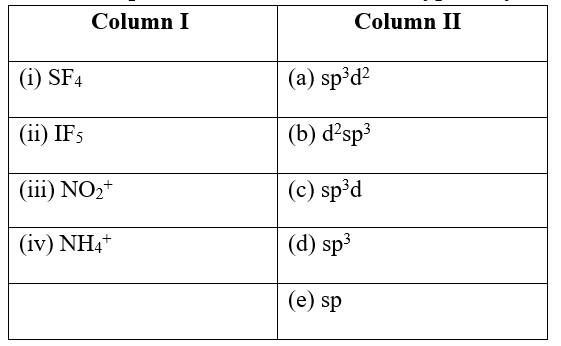
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) SF4 (c) sp3d
(ii) IF5 (a) sp3d2
(iii) NO2+ (e) sp
(iv) NH4+ &nbs
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and option (ii)
(i) NaCl being an ionic compound is a good conductor of electricity in the molten state. It is not a bad conductor of electricity in solid state. So, the statement mentioned in the question is not correct. Hence, this is the correct answer.
(ii) There is no difference in the arrangement of atoms in the canonical structures. The difference is in the arrangement of electron pairs in the canonical structures. So, the statement mentioned in the question is not correct. Hence, this is the correct answer.
(iii) The hybrid orbitals have the same
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers