Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Get insights from 99 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Four, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Four
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii) and (iv)
The formula of bond order is:
Bond order = ( Nb- Na)
Number of bonding and antibonding electrons can be found from the molecular electronic configuration of the species.
(i) Total number of electrons in N2 is 14. So, its molecular electronic configuration will be
σ1s2 σ∗1s2 σ2s2 σ∗2s2, π2p2x , π2p2y, s2pz2
The bond order will be:
( 10-4)= 3
(ii) The total number of electrons in N2 - is 15. So, its molecular electronic configuration will be:
σ1s2 σ∗1s2 σ2s2 σ∗2s2, π2p2x , π2p2y, s2pz2 π*2p1x
The bond order will be:
( 10-5)=
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and (iv)
(i) N2 will have 14 electrons in its structure. So, its electronic configuration will be:
σ1s2 σ∗1s2 σ2s2 σ∗2s2, π2p2x , π2p2y, s2pz2
According to the molecular electronic configuration of N2 it does not contain any unpaired electrons, it will be diamagnetic in nature.
(ii) N22– will have 16 electrons in its structure. So, its molecular electronic configuration will be:
σ1s2 σ∗1s2 σ2s2 σ∗2s2, π2p2x , π2p2y, s2pz2, , π*2p1y
According to the molecular electronic configuration of 2 N2 -, it has two unpaired electrons in π*2p1x ,
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii) and (iv)
(i) The hybridization of the central carbon atom in CO3 2- is 2 sp as the carbon is bonded with three oxygen atoms. So, the given statement is incorrect.
(ii) The resonance structure of CO32- has one C= O double bonds and two C- O single bonds. So, the statement is incorrect.
(iii) The net charge on three oxygen atoms is -2, hence the average formal charge on each oxygen atom is 0.67 units. So, the given statement is correct.
(iv) As it can be seen from the resonating structures that the structures of the bonds are not fixed. Hence, all the C-
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii) and (iv)
(i) The number of valence electrons in CO2 is 14. The oxygen atom attached to the carbon atom forms a double bond. Therefore, the hybridization of the structure is sp2. Thus, carbon dioxide is linear in shape.
(ii) The number of valence electrons in CCl4 is 32. The structure of CCl4 is arranged in a tetrahedral manner. The chlorine atoms are arranged such that the hybridization is sp3.
(iii) The number of valence electrons in O3 is 24. The hybridization of the molecule is sp2 and due to the presence of lone pair of electrons on the central oxy
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and (ii)
The species having the same number of electrons are known as isoelectronic species.
The number of electrons in CO species is 14; 8 electrons from oxygen atom and 6 electrons from carbon atom.
The number of electrons in NO+ is 14; 8 electrons from oxygen atom, 7 electrons from nitrogen atom and 1 electron will be subtracted due to the positive charge on the species.
The number of electrons in N2 is 14 ; 7 from each nitrogen atom.
The number of electrons in SnCl2 is 84 ;50 from the tin atom and 17 from each chlorine atom.
The number of electrons in NO2
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and (iv)
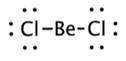
The structures of NO2 and NCO+ has a lone pair of electron on nitrogen atom thus it will form a bent shape rather than a linear shape.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i) and option (ii)
Bond order depends on the electronic configuration of the molecular species, and the electronic configuration depends on the number of electrons a particular atom is contributing in its molecular structure.
The CN- species has a total of 14 electrons; 6 electrons from carbon atom, 7 electrons from nitrogen atom and 1 electron for the negative charge.
The NO+ species has a total of 14 electrons; 8 from oxygen atoms, 7 from nitrogen atom and 1 electron will be subtracted due to the positive charge on the species.
The O2 - species has a total
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
Aluminium has [Ne]3s2 3p1 electronic configuration. Phosphorus has [Ne]3s2 3p3. The electronic configuration of silicon is [Ne]3s2 3p2 and the electronic configuration of arsenic is [Ar]3d104s24p3.
Ionisation energy increases as the atomic number in a period increases and decreases on moving down the group. It is also known that the ionisation enthalpy of group 15 elements is more than group 16 elements as group 15 has half-filled p-subshells which gives extra stability.Thus, the electronic configuration having the highest ionization entha
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
The most electronegative element in the periodic table is fluorine having an atomic number 9 . The electronic configuration of fluorine is 1s2 2s2 2p5. Hence, the electronic configuration of the outermost shell of fluorine is 2s2 2p5 .
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
The electronic configurations and the bond orders of the given species are given below:
Bond order of O2 - : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p1y)
Bond order = ( Nb- Na)
BO= (10-7)= = 1.5
Bond order of O2 + : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p0y)
Bond order = (10-5)= =2.5
Bond order of O2 : σ1s2 σ∗1s2 σ2s2 σ∗2s2 σ2p2z (π2p2x = π2p2y) (π∗2p1x= π∗2p1y)
BO= (10-6) = =2
So, the correct order of bond order is (ii) O2 – < O2 < O2 +
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
