Chemistry NCERT Exemplar Solutions Class 12th Chapter One
Get insights from 118 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter One, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter One
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
67. Options (ii) and (iv) are correct since Vacancy and Schottky, both the defects lead to the decrease in the density while in the Frenkel and Interstitial defect there is no change in the density of the substance.
New answer posted
7 months agoContributor-Level 10
66. Options (i) and (ii) are correct since in this defect an atom is displaced from its lattice point to an interstitial site and thus creating a vacancy at the lattice point. This usually occurs when the size of anion is quite large as compared to that of the cation due to which it leads to the dislocation of the cation to the interstitial site and hence it is known as dislocation defect and during this defect there is no change in the stoichiometry of the crystal so, it is also known as stoichiometric defect.
New answer posted
7 months agoContributor-Level 10
65. Options (i) and (iv) are correct since when the tetrahedral void of the first layer and the tetrahedral void of the second layer align together, they form an octahedral void, and this is possible in case of hep and fcc arrangements.
New answer posted
7 months agoContributor-Level 10
64. Options (i), (ii) and (iii) are correct since SiC, AlN, and diamond are not regarded as molecular solids, instead they come under network or covalent solids.
New answer posted
7 months agoContributor-Level 10
63. Options (i) and (iii) are correct since quartz glass is an amorphous solid so, it does not exhibit a definite heat of fusion.
New answer posted
7 months agoContributor-Level 10
62. Options (i) and (iv) are correct since these statements agrees with the ferrimagnetic and ferromagnetic nature of the substances respectively.
New answer posted
7 months agoContributor-Level 10
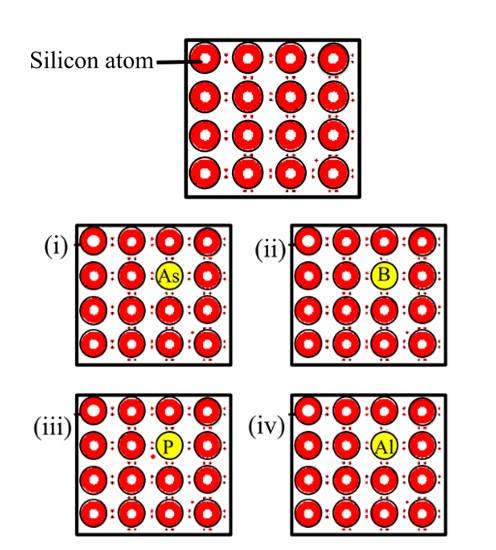
61. Options (i) and (iii) are correct since n-type semiconductors are prepared by doping silicon with pentavalent elements of group 15 (like P or As). Some of the lattice sites in silicon crystals are occupied by the fifth electron which is left over as extra electron and couldn't form a bond with the tetravalent Silicon and hence the images (i) and (iii) are showing this situation and hence representing n-type semiconductor.
New answer posted
7 months agoContributor-Level 10
60. Options (i) and (iii) are correct since the structure of the amorphous solids is similar to that of the liquids and hence they tend to flow like liquids, although very slowly. So, sometimes they are known as super cooled liquids or pseudo solids.
New answer posted
7 months agoContributor-Level 10
59. Options (ii) and (iii) are correct since the number of tetrahedral voids per unit cell in NaCl is calculated as-
Since NaCl crystal forms fcc lattice so,
The number of atoms (n) per unit cell = 4
So, the number of octahedral voids = n = 4
While the number of tetrahedral voids = 2n = 2*4=8
New answer posted
7 months agoContributor-Level 10
58. Options (i) and (iv) are correct since an excess of K+ ions make KCl crystals appear violet due to the defect called metal excess defect . The anionic vacancies are found to be created due to the movement of potassium atoms to the surface of the crystal when KCl is heated in an atmosphere of potassium vapours and the Cl-ions then diffuse to the surface.
This happens due to the loss of the electron by the potassium atom to form K+ ions. In this case the electrons released diffuse to the crystal and occupy anionic sites being created. The anionic sites occupied by unpaired electrons are known as F-centres. These F-centres impart
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers