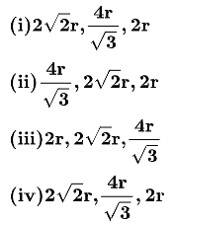Chemistry NCERT Exemplar Solutions Class 12th Chapter One
Get insights from 118 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter One, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter One
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
57. Options (ii) and (iii) are correct since silicon has 4 valence electrons and is doped with electron rich impurity is an n-type semiconductor due to extra electrons and hence the availability of the delocalised electrons increase the conductivity of this.
New answer posted
7 months agoContributor-Level 10
56. Options (i) and (ii) are correct since Si or Ge of group-14 are doped with electron deficient impurity i.e., group-13 elements like B, Al, or Ga containing only three valence electrons and hence is called as p-type semiconductors while the electron holes are created whenever an atom of such trivalent metal gets bonded with three of its neighbouring tetravalent silicon or germanium atoms.
The fourth valence electron of Ge or Si moves through the holes and under the influence of the electric field electrons move towards the positively charged plate through these holes. But it would appear as if the electron holes are posit
New answer posted
7 months agoContributor-Level 10
55. (i), (ii) and (iv) is correct since gap between valence band and conduction band determines the conductivity of the metals. The electrical conduction of metals depend upon the type of valence band & also the gap between the valence band and conduction band while the valence band may remain partially filled.
New answer posted
7 months agoContributor-Level 10
54. Options (iii) and (iv) are the statements which are not true since in impurity defect there is a replacement of ions by the ions of another compound. By doing so, even though the electrical neutrality of the solid is maintained, a vacancy defect is caused due to creation of vacant sites in the ionic solid. This results into change in density of the solid. For example, if molten NaCl containing a little amount of SrCl2 is crystallised some of the sites of Na+ are occupied by Sr2+ .
Each Sr2+ replaces two Na+ ions and hence this affects density. Frenkel defect causes vacancy defect at its original site and an interstitial d
New answer posted
7 months agoContributor-Level 10
53. Options (iii) and (iv) are correct since in anti-ferromagnetic substances the magnetic moments and domains are oppositely oriented, and they cancel out each other's magnetic moment. For example, the anti-ferromagnetic substance-MnO.
New answer posted
7 months agoContributor-Level 10
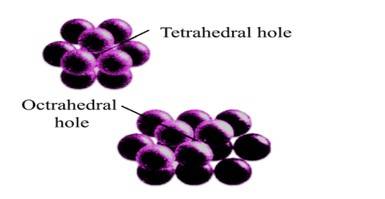
52. Option (iii) and (iv) are correct since tetrahedral voids are formed when the triangular void in the second layer lie exactly above the triangular voids in the first layer and the triangular shape of these voids oppositely overlap while the octahedral voids are formed when triangular void of second layer is not exactly overlap with similar void in first layer. This can be shown as-
New answer posted
7 months agoContributor-Level 10
51. Option (i)? metals >>? insulators < ? semiconductors is correct since metals are the ones which shows the highest conductivity due to the presence of free electrons in them which helps in the conduction of electricity while semiconductors are the ones whose conductivity depends upon the temperature.
Since at normal temperatures, they act as insulators whereas if the temperature rises, the conductivity of the semiconductor also increases. Insulators are the ones which do not have free electrons in them. Due to which they act as poor conductors and hence their conductivity is the lowest.
New answer posted
7 months agoContributor-Level 10
50. Option (i) , ,2r
In FCC crystal - the atoms touch each other at the face diagonals. If a is the edge length of the cube and r is the radius of the atom
Therefore,
a =
In a simple cubic crystal - The atoms at the corners touch each other.
Therefore, a = 2r
New answer posted
7 months agoContributor-Level 10
49. Option (iv) 8 tetrahedral voids within the unit cells is correct since in ccp structure there are 4 atoms per unit cell and the number of tetrahedral voids is twice the number of atoms (i.e. eight tetrahedral voids per unit cell of cubic close packing.
New answer posted
7 months agoContributor-Level 10
48. Option (i) Frenkel defect is correct since in this defect an atom is displaced from its lattice point to an interstitial site thus creating a vacancy at the lattice point. This usually occurs in those ionic crystals where the size of anion is quite large as compared to that of the cation. Here dislocation of atoms occurs and hence it is known as dislocation defect.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers