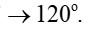Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
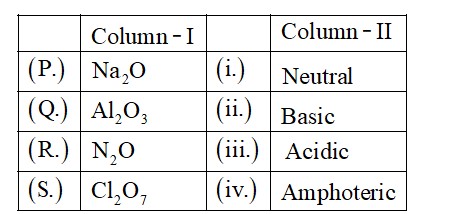
Group?17, elements have very high ionisation enthalpy due to increase in atomic size, ionization enthalpy decreases down the group.
New answer posted
3 months agoContributor-Level 10
Sodium carbonate is a white crystalline solid which exists as a decahydrate, 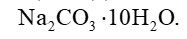
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
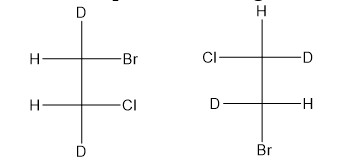
Bond angles give some idea regarding the distribution of orbitals around the central atom in a molecule/complex ion and hence it helps us in determining its shape
New answer posted
3 months agoContributor-Level 10
Dv = a (t + 1 – t)
a = 50 m/s2
u = 120 – 25 (2t +1)
In (t + 2)th second
= u + 25 (2t + 3)
= 120 – 25 (2t) – 25 + 25 (2t) + 75
S' = 170 m
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers