Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
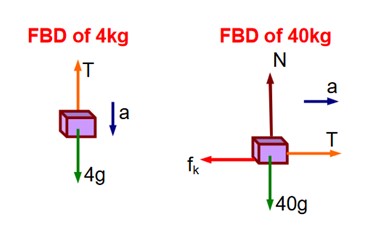
According to Newton's laws of motion, we can write
4a = 4g – T . (1), and
40a = T - fk = T (40g) ……………… (2)
Adding equations (1) and (2), we can write
44a = 40 – 0.02 * (400) = 32
New answer posted
5 months agoContributor-Level 10
According to question, we can write
Total moles of gas = n = nOxygen + nOxygen =
Volume of gas = 12 * 22.4 litre = 268.8 litre = 2.688 * 105 cm3
New answer posted
5 months agoContributor-Level 10
According to question, we can write
Increment in height of tower = h2 – h1 = 500 – 125 = 375 m
New answer posted
5 months agoContributor-Level 10
According to question, we can write
Increment in height of tower = h2 – h1 = 500 – 125 = 375 m
New answer posted
5 months agoContributor-Level 10
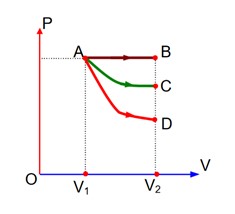
Process-AB Isobaric,
Process-AC Isothermal, and
Process-AD? Adiabatic
W2 < W1 < W3
New answer posted
5 months agoContributor-Level 10
Each element of ordered pair (i, j) is either present in A or in B.
So, A + B = Sum of all elements of all ordered pairs {i, j} for and
= 20 (1 + 2 + 3 + … + 10) = 1100
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers