Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Sum of all elements of [Sum of natural number upto 100 which are neither divisible by 3 nor by 5]
= 10100 – 3366 – 2100 + 630
= 5264
New answer posted
5 months agoContributor-Level 10
Let y = mx + c is the common tangent
so equation of common tangents will be
which intersects at Q (3, 0)
Major axis and minor axis of ellipse are 12 and 6. So eccentricity
and length of latus rectum
Hence
New answer posted
5 months agoContributor-Level 10
Boys (10) Girls (5)
(3) (3)
B1 & B2 should not be selected together
Total number of ways
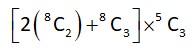
= (56 + 56) * 10 = 1120
New answer posted
5 months agoContributor-Level 10
So, f (x) is decreasing function and range of f (x) is
which is
Now 4a – b = 4 ( + 5) (5 + 9) = 11 - π
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers