Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Let v1 and v2 are velocities of two balls after collision
According to conservation of momentum
2mvo = mv1+ mv2
2vo= v1+v2
and e= v2-v1/2vo
v2=v1+2voe
2v1=2vo-2evo
V1=vo (1-e) since e<1 so ball will move after collision.
b)by principle of conservation of linear momentum

P=P1+P2
For inelastic collision some KE is lost hence >
P2>p12+p22
Thus P, P1 and P2 are related as shown in fig
P2>p12+p22 this condition only holds when angle is 90.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
mechanical energy =KE+PE
Eo = KE + V (x)
KE= E0 -V (x)
At A x=0 v (x)=Eo
KE= Eo-Eo =0
atB, V (x)
so KE>0
at C and D, V (x)= 0
KE is maximum at FV (x)= Eo

Hence KE= 0
As KE= 1/2mv2
Therefore at A and F where KE =0, v=0

At C and D KE is maximum therefore v is maximum.
At B KE is positive but not maximum but it has some value.
New answer posted
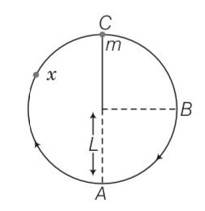
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
At B the velocity of B is vertically downward, therefore when string is cut at B then it fall downwards.
At C velocity along horizontally right, so when we cut it at C then it will move to right. But under the action of gravity its path becomes parabola.
At X when we cut it moves tangentially in forward direction. So under of the action of gravity it also follows parabola path.

New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
According to work energy theorem = change in kinetic energy = work done
Kinetic energy of the body = force (displacement)
As kinetic energy is same so both will move with same velocities.
New answer posted
7 months agoGive example of a situation in which an applied force does not result in a change in kinetic energy.
Contributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
When charge particle moves in a uniform magnetic field the path of particle is circular . so when it moves in circular path its radius Is also constant . so its kinetic energy also constant.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Average work done by a human heart per beat =0.5J
Total work done during 72 beats= 72 =36J
Power = work done/time
=36/60=0.6W
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Total linear momentum of the system of two balls is always conserved. While balls are in contact, there may be deformation which means elastic PE which came from part of KE .But they are in contact while collision so according to above case kinetic energy will not conserved.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
No, work done is not always zero in circular path it is zero only when all the forces are zero.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
No total mechanical energy of the body falling freely under gravity is not conserved because a small part of its energy is utilised against resistive force of air, which is non conservative force. Gain in KE< loss in PE.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Force of gravity acts on the car vertically downward while car is moving along horizontal direction. So angle between them is 90. So work done is zero.
W= fscos90=0
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers