Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Force between two protons is same as that of between proton and a positron. As positron is much lighter than proton, it moves away through much larger distance compared to proton.
We know that work done = force (distance). As forces are same in case of proton and positron but distance moved by positron is larger, hence work will be more.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) When electron and proton are moving under influence of their mutual forces the magnetic forces will be perpendicular to their motion hence no work is done by these forces.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Energy given by 1 lit of petrol = 3
Efficiency of the car engine=0.5s
Energy used by the car = 0.5
Total distance travelled s = 15km = 15
If f the force of friction= E= f
1.5 = f
F=
F= 1000 N
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Weight of the adult w= mg =600N
Height of each step = h = 0.25m
Total distance travelled = 6km =6000m
Total number of steps = 6000/1= 6000
Total energy utilised in jogging = n mgh
= 6000 600 0.25J
= 9 J
Since 10% of intake energy is utilised in jogging
So total energy intake = 10
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Mass of the system = 50000kg
Speed of the system v= 36km/h= 10m/s
Compression of the spring x= 1m
KE of the system = 1/2mv2
= ½ (50000) (10)2
= 25000 (100)J= 2.5
Since 90 % of KE is lost due to the friction so energy transferred is
E= 1/2kx2= 10% of total KE of the system
= 0r K =
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Mass of drop m = 3
Terminal velocity = 9m/s
Height = 100cm=1m
Density of water = 103kg/m3
Area of the surface = 1m2
Volume of the water due to rain V = area height
= 1 (1)= 1m3
Mas s of water due to rain M= volume (density)
= V ( )= 103kg
Energy transferred to the surface = 1/2mv2
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
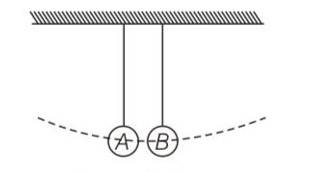
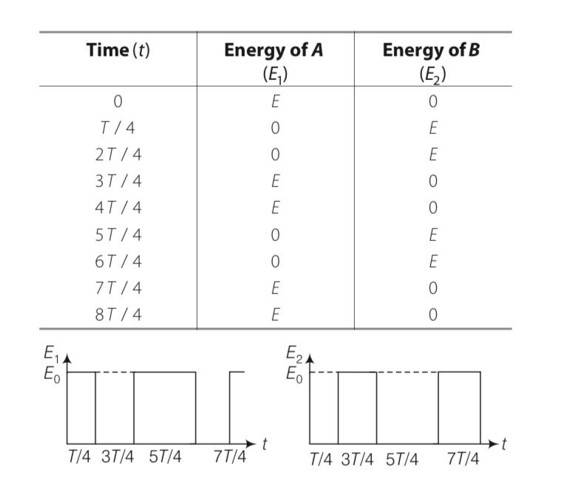
At t=0 suppose bob B is displaced by angle 10 to the right . it is given potential energy E1=E . energy of A, E2=0
When B is released it strikes at A at t=T/4 in the head on elastic collision between B and A comes to rest and A gets velocity of B. therefore E1=0 and E2=E. at A =2T/4, B reaches its extreme right position when KE of A is converted into PE=E2=E . Energy of B, E1=0
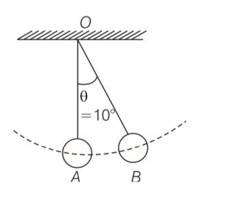
At t=3T/4. A reaches its mean position when its PE is converted into KE =E2 =E. it collides elastically with B and transfers whole of its energy to B. thus E2=0 and E1 =E . the entire process is r
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
mass of the rain drop = 1g=1
Height of falling h= 1km = 103m and g = 10m/s2 and sped of drop =50m/s
(a) Loss of PE of the drop =mgh= 1
(b) Gain in KE of the drop = 1/2mv2= ½ =1.250J
(c) No gain in KE is not equal to the loss in its Pe, because a part of PE is utilised in doing work against the viscous drag in air.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
When the ball A reaches bottom point its velocity in horizontal direction
(a) two balls have same mass and the collision between them is elastic therefore ball A transfers its entire linear momentum to ball B. hence ball A will come to rest after collision and does not rise at all.
(b) speed at B = speed with which A hits the ball B
=
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
TE=KE+PE
E=V+K
For region A given V>E
so 'K=E-V
V>E
So E-V <0
Hence K<0 this is not possible.
For region B given V
So E-V>0
This is only possible because total energy is greater than PE
For region C given K>E
So K-E>0
So PE =V= E-K <0
Which is possible because PE can be negative.
For region D given V>K
This is possible because for the system PE may be greater than KE
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers