Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoBeginner-Level 5
Human Physiology is fifth unit in NCERT book of class 11th. It comprises total six topics, covering various aspects of digestion, respiration, circulation, excretion, and locomotion.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
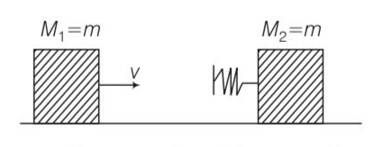
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) conserving energy between “O” ans ”A”
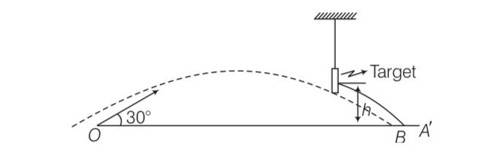
Ui + Ki = Uf + Kf
0+1/2mv2= mgh + 1/2mv'
(v')2=v2-2gh = v'= ……….1
Let speed after emerging be v1 then
=1/2mv12=1/2[1/2mv'2]
1/2m(v1)2=1/4m(v')2=1/4m[v2-2gh]
V1= ………….2
From eqn 1 and 2
So v1 = v'/ =v2(v'/2)
v1>v'/2
hence after emerging from the target velocity of the bullet is more than half of its earlier velocity v'
(d) as the velocity of the bullet changes to v' which is less than v' hence , path, followed will change and the bullet reaches at point B instead of A
(f) as the bullet is passing through the target
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b, d) When a man of mass m climbs up the staircases of height L, work done by the gravitational force on the man is mgl work done by internal muscular forces will be mgL as the change in kinetic is almost zero.
Hence total work done =-mgL + mgL=0
As the point of application of the contact forces does not move hence work done by reaction forces will be zero.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) m =150g =3/20kg
Time of contact =0.001s
U=126km/h=
V= -35m/s
Change in momentum of the ball = m (v-u)=
=21/2
F= dp/dt=- = - 1.05
Here – negative sign indicates that force will be opposite to the direction of movement of the ball before hitting.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) First velocity of the iron sphere increases and after sometimes becomes constant called terminal velocity. Hence according first KE increases and then becomes constant which is best represented by b.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(d) h =15m, v= 1m/s, m= 10kg, g= 10m/s2
From conservation of mechanical energy
(PE+KE)initial= (PE+KE)final
Mgh + =0+KE
KE= mgh +
KE= 10
KE= 150+5=155J
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) When drop falls first velocity increases, hence first KE also increases, after sometime speed is constant this is called terminal velocity, hence KE also become constant PE decreases continuously as the drop is falling continuously . the variation in PE and KE is best represented by b.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a) Mass m = 5kg
Radius =1m=R
Revolution per minute w= 300rev/min
= 300 ( )rad/min
=
Linear speed v= rw
=
KE= 1/2mv2
= 2J
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) When a pendulum oscillates in air, it will lose energy continuously in overcoming resistance due to air. Therefore total mechanical energy of the pendulum decreases continuously with time. This variation is correctly represented by option c.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers