Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
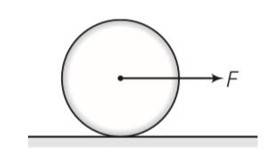
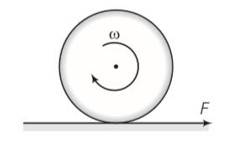
Frictional force f is acting in the opposite direction of F . let the acceleration of centre of mass of disc be a then
F-f=Ma where M is the mass of the disc
fR= (1/2 MR2)
so fR= (1/2MR2) (a/R)
Ma=2f
From the above equation
F = F/3
F< =
f/3 <
F=3
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
(A) Carbon has small size and large electronegativity, it forms strong n−pπ bonding with two oxygen atoms forming a separate CO2 molecule.
While in SiO2 silicon is larger in size with comparatively less electronegativity than carbon it shows no tendency to form n−pπ bonding rather forms Single covalent bond with oxygen. Thus, SiO2 possess 3D network-like structure in which each Silicon is bonded to 4 oxygen atoms.
(B) Carbon is smaller in size and lacks d-orbitals hence can have a maximum coordination number of four and sp3 hybridisation only.
Wherea
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
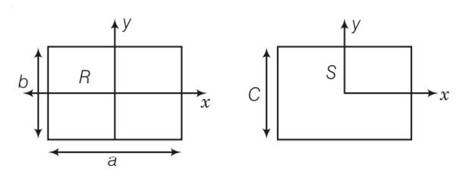
area of square = area of rectangular plate
C2=a b
(a)
here bxR
(b) here a>c so IyR>IyS
(c) IzR-IzS +2ab= (a-b)2
(IzR-IzS)>0
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
(A) sarbon in CCl4 does not have a vacant d-orbital to accommodate the electrons from OH of water molecules. Also CCl4 is nonpolar covalent compounds whereas H2O is polar. So, no strong interaction occurs between them. Hence CCl4 is miscible in water.
Whereas in SiCl4, silicon has bigger size than carbon and have d-orbitals for accommodation of electrons donated by OH of water in the process of hydroxylation. This leads to a strong interaction and silicon acid Is formed as a product. SiCl4 is completely miscible in water.
(B) As we move from carbon to sil
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Both BCl3 and AlCl3 are electron deficient compounds that are central atom boron and aluminium have incomplete Octet. In each compound, a metal atom is surrounded by six electrons of three covalent bonds with 3 chlorine atoms.
Each chlorine atom has a complete Octet of eight electrons. The electron deficient compounds act as Lewis acid and readily accept two electrons to complete their octet.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) Situation as given below
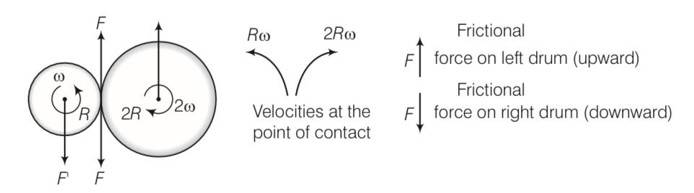
(b) F' =F=F'' where F' and F'' are external forces through support.
So Fnet=O
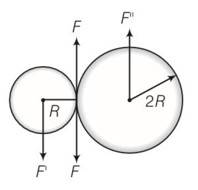
External torque = F (anticlockwise)
(c) Let w1 and w2 be the final angular velocities of smaller and bigger drum in both clockwise and anticlockwise . finally there will be no friction
hence Rw1=2Rw2
so
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
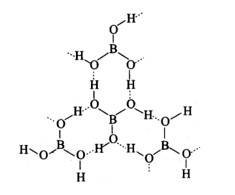
H3BO3
Boric acid forms a hexagon and rings through hydrogen bonding and has a layer-like structure.
Boric acid is present in water as [B (OH)4]−.H3BO3 electron from the OH of water and forms the complex BOH for negative for sp3 and is present in sp3 hybridisation.
Reaction:
B (OH)3+2H2O→ [B (OH)4]− + H3O+
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) Before being bought in contact with the table the disc was in pure rotational motion. Hence Vcm=0
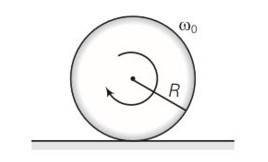
(b) when the disc is placed in contact with table due to friction centre of mass acquires some linear velocity.
(c) when the rotating disc is placed in contact with the table due to friction centre of mass acquires some linear velocity.
(d) friction is responsible for the effects ib b and c
(e) when rolling starts Vcm=wR
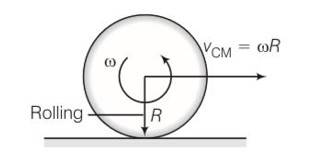
(f) time period for rolling to begin is t=
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) yes the law of conservation of angular momentum can be applied because there is no net external torque on the system of the two discs.
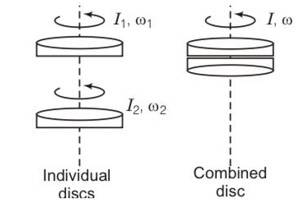
External forces gravitation and normal reaction act through the axis of rotation, hence produce no torque.
(b) by conservation of angular momentum
Lf= Li
Iw=I1w1+I2w2
So w=
(c) Kf=
Kf= ½ (I1w12+I2w22)
Kf-Ki =- 2<0
(d) hence there is loss of KE of the system. The loss of kinetic energy is mainly due to work against the friction.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
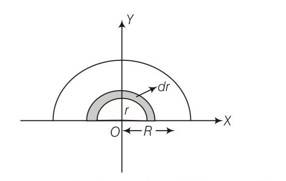
let M and R be mass and radius of half disc , mass per unit area of half disc
So m =
(a) the half disc be supposed to be consist of a large number of semicircular ring of mass dm and thickness dr and radii ranging from r=0 to r=R.
surface area of semicircular ring of radius r and thickness dr =
so mass of the elementary ring dm =
dm=
if x,y are coordinates of centre of mass of this element,
then (x,y)=(0,2r/ )
so x=0 and y =2r/
let xcm and ycm be the coordinates of the centre of mass of the semicircular disc
so xcm=
Ycm=
= 0R
= 4R/3
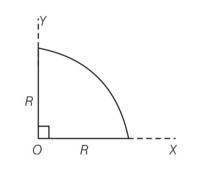
Centre of mass of a uniform quar
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers