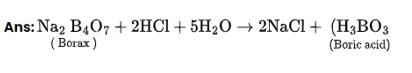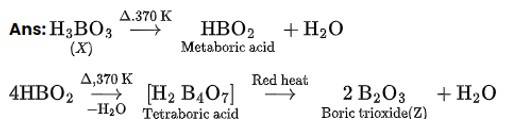Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
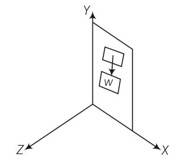
Where weight of the door acts along negative y axis
A force can produce torque only along a direction normal to itself as . So when the door is in the xy plane the torque produced by gravity can only along z direction. Never about an axis passing through y direction. Hence the weight will not produce any torque.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
A
Central atom in [B (OH)4 ]− no lone pair. Electronic conf In ground state: 1s2 2s2 2px1 2py0 2pz0
In excited state, One Electron from 2s shifts to 2py orbital and the configuration becomes:
1s2 2s2 2p1 x2 p1 y2 pz0
Now, one s and three p orbitals combined to give sp3 hybridisation and tetrahedral shape.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
A
Lewis acids are the species in which the state is not complete and ready to accept electrons. Because Al is surrounded by 6 electrons in AlCl3 and all three Cl atoms are surrounded by 8 electrons, AlCl3 is an electron acceptor. It is a covalent compound.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Wheel is a rigid body. The particles that constitute the wheel do experience a centripetal acceleration directed towards the centre. This acceleration arises due to internal elastic forces which cancel out in pairs.
In a half wheel the distribution of mass about its centre of mass is not symmetrical, therefore the direction of angular momentum of the wheel does not coincide with the direction of its angular velocity. Hence an external torque is required to maintain the motion of the wheel.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
C
Gallium generally exists as solid at room temperature but melts on slight heating (melting point: 20? C ).
Whereas the boiling point of Gallium is very high around 2400? C. Gallium has large cohesive forces that hold its structure together and it is stable for a wide range of temperatures and can be used for measuring high temperatures.
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
4BF3 + 3LiAlH4? 2 B2H6 + 3LiF + 3AlF3
(Z) (X)
B2H6 + 6H2O?2H3BO3 + 6H2
(X) (Y)
New answer posted
7 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
(i) When moving down the periodic table within a group, ionization enthalpy generally decreases due to increased atomic radius. However, in the case of gallium (Ga) and aluminum (Al), gallium experiences a higher effective nuclear charge due to less shielding from its inner electrons, resulting in a higher ionization enthalpy compared to aluminum.
(ii) As boron is smaller in size and the sum of its first three ionization enthalpies i.e. ΔH1+ΔH2+ΔH3 is very large so boron does not allow to lose its all three valence electrons and exist as +3
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
no
The sum of torques about a certain point O
The sum of torques about any other O
The sum of torques about any other point O'
Here the second term need not vanish.
Sum of all torques about any point is zero.
New question posted
7 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers