Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
11.18 Base area of the boiler, A = 0.15 m2
Thickness of the boiler, l = 1.0 cm = 0.01 m
Boiling rate of water, R = 6.0 kg/min
Let us assume the mass of the boiling water, m = 6 kg and the time to boil, t = 1 min = 60 s
Thermal conductivity of brass, K = 109 J s–1 m–1 K–1
The amount of heat flowing into water through the brass base of the boiler is given by:
= , where
= Flame temperature in contact with the boiler
= Boiling point of water = 100
Heat required for boiling water = mL, where L = heat of vaporization of water = 2256 * 103 J kg–1
By equating for we get
6 = 109&n
New answer posted
8 months agoContributor-Level 10
No, Bernoulli's equation cannot be used to describe the flow of water through a rapid in a river. In rapid, the flow is turbulent whereas Bernoulli's equation is applicable for laminar flow only.
New answer posted
8 months agoContributor-Level 10
11.17 Size of the sides of cubical ice box, s = 30 cm =0.3 m
Thickness of the icebox, l = 5 cm = 0.05 m
Mass of ice kept in the box, m = 4 kg
Time gap, t = 6 h = 6 s
Outside temperature, T = 45 °C
Coefficient of thermal conductivity of thermocole, K = 0.01 J
Heat of fusion of water, L = 335 J
Let m' be the mass of the ice melts in 6 h
The amount of heat lost by the food: = , where
A = Surface area of the box = 6 = 6 = 0.54
= = 104976 J
We also know so m' = 104976/ (335 = 0.313 kg
Hence the amount of ice remains after 6 h = 4
New answer posted
8 months agoContributor-Level 10
Height of the spirit column, = 12.5 + 15 cm = 27.5 cm
Height of the water column, = 10 + 15 cm = 25 cm
Density of spirit, = 0.8 gm/
Density of water, = 1 gm/
Density of mercury, = 13.6 gm/
Let h be the difference between the levels of mercury in two limbs
Pressure exerted by mercury column of h height= h = 13.6hg …. (i)
Difference between pressure exerted by water and spirit columns:
= 3g ……. (ii)
Equating equations (i) and (ii), we get
13.6hg = 3g
h = 3/13.6 = 0.221 cm
New answer posted
8 months agoContributor-Level 10
11.16 Initial body temp of the child, = 101°F
Final body temp of the child, = 98°F
Change in temperature, T = 98°F) = 3 °F = (3-32) C = 1.666
Time taken t achieve this temperature, t = 20 min
Specific heat of human body = Specific heat of water, c = 1000 cal/kg/
Latent heat of evaporation of water, L = 580 cal/g
Mass of the child, m = 30 kg
The heat lost by the child is given as = 30 = 49980 cal
Let be the mass of water evaporated from the child's body in 20 mins.
Loss of heat = = 580 =
&nb
New answer posted
8 months agoContributor-Level 10
Height of the spirit column, = 12.5 cm = 0.125 m
Height of the water column, = 10 cm = 0.1m
= atmospheric pressure
= density of spirit, = density of water
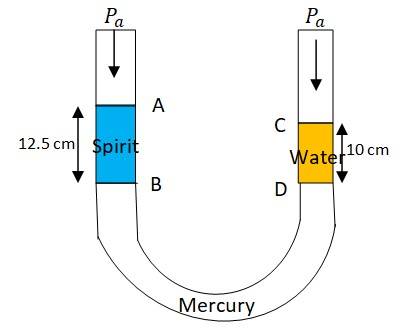
Pressure at point B = +
Pressure at point D = +
But pressure at point B and D are same. Hence
+ = + or =
= = = 0.8
So the specific gravity of spirit is 0.8
New answer posted
8 months agoContributor-Level 10
11.15 The gases listed above are diatomic. Besides the translational degree of freedom, they have other degrees of freedom. Heat must be supplied to increase the temperature of these gases. This increases the average energy of all the modes of motion. Hence the molar specific heat of diatomic gases is more than that of monatomic gases.
If only rotational mode of motion considered, then the molar specific heat of a diatomic gas
= R = = 4.95 cal mo1–1 K–1
With the exception of Chlorine, all the observations given above agrees with ( R). This is because at room temperature, chlorine also has vibrational modes
New answer posted
8 months agoContributor-Level 10
The maximum mass of the car can be lifted, m = 3000 kg
Area of cross-section of the load carrying piston, A = 425 = 425
Maximum force exerted by the load, F = mg = 3000 N = 29400 N
Maximum pressure exerted, P = F/A = (29400 / 425 ) Pa = 6.917 Pa
New answer posted
8 months agoContributor-Level 10
11.14 Mass of the metal, m = 0.20 kg = 200 g
Initial temperature of the metal, = 150 °C, Final temperature of the metal, = 40 °C
The water equivalent mass of the calorimeter, m' = 0.025 kg = 25 g
Volume of water, V = 150 cm3
Mass of water, M at T = 27 °C, = 150
Fall in metal temperature, T = = 150 – 40 = 110 °C
Specific heat of water, = 4.186 J/g/ K
Let the specific heat of metal = C
Then, heat loss by the metal, = mC T ……. (i)
Rise in the water of the calorimeter system T' = 40 – 27 = 13°C
Heat gained by the water and calor
New answer posted
8 months agoContributor-Level 10
The maximum allowable stress for the structure, P = Pa
Depth of the ocean, d = 3 km = 3 m
Density of water = kg/
Acceleration due to gravity, g = 9.8 m/s
The pressure exerted because of sea water at the depth d = = 3 Pa
= 2.94 Pa
The maximum allowable stress is more than the pressure, hence the structure is suitable.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
