Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
11.8 Initial temperature, = 27.0 °C, Initial diameter of the hole, = 4.24 cm
Final temperature, = 227.0 °C, Final diameter of the hole
Coefficient of linear expansion of copper, = 1.70 K–1
We know
= T where is the coefficient of superficial expansion,
= T
= T
- 1= 2 1.70 (227-27) = 6.8
= 4.2544 cm
So change in diameter = 4.2544 – 4.24 = 0.01439 cm
Diameter increase by 1.44 cm
New answer posted
8 months agoContributor-Level 10
The pressure of a liquid is given by the following relation:
P = h g, where P = Pressure, h = height of the liquid column, = is the density of the liquid and g= acceleration due to gravity
From the above relation, because of the h factor (height of the human body), the pressure is more at the feet and less at the brain
The said phenomenon is due to the factor. Density of air is maximum at the sea level. At height, density decreases and pressure also decreases. At 6 km height, the density of air is nearly half of that of a t sea level
When pressure is applied on the liquid, the pressure is transmitted in all dir
New answer posted
8 months agoContributor-Level 10
11.7 Given, temperature = 27 °C = 27 + 273.16 K = 300.16 K
Outer dia of the shaft at temp , = 8.7 cm
Diameter of the central hole of the wheel, = 8.69 cm
The change in diameter, Δd= 8.69 – 8.7 = 0.01 cm
After the shaft is cooled in dry ice, its temperature becomes . It can be calculated from the relation
Δd= (
-0.01 = 8.7
= -95.78
= 204.22 K = -68.94
New answer posted
8 months agoContributor-Level 10
11.6 Length of the steel tape, l = 1 m = 100 cm, At temperature T = 27 C
Coefficient of linear expansion of steel = 1.2 / K
Let be the length of the steel rod at temperature = 45.0 °C and
be the length of the steel rod and l' be the length of the steel tape at 45.0 °C
We have l' = l + = 100 + 1.2 (45-27) = 100.0216 cm
can be calculated as = = 63.0136 cm
New answer posted
8 months agoContributor-Level 10
11.5 (a) For Thermometer A
Triple point of water, T = 273.16 K
At this temperature, the pressure in thermometer A , = 1.250 Pa
Let be the temperature for the normal melting point of sulphur and be the corresponding pressure. It is given, = 1.797 Pa
From Charles' law, we get = , = = = 392.69 K
For Thermometer B
Triple point of water, T = 273.16 K
At this temperature, the pressure in thermometer B , = 0.2 Pa
Let be the temperature for the normal melting point of sulphur and be the co
New answer posted
8 months agoContributor-Level 10
11.4 (a) The triple point of water has a unique value of 273.16 K, irrespective of pressure and volume. Whereas, melting point of ice and boiling point of water, the temperature value depends on pressure and volume.
(b) The other fixed point on Kelvin scale is 0 K.
(c) The temperature 273.16 K is the triple point of water, it is not the melting point of ice. The melting point of ice is specified in Celsius scale as 0 Hence the absolute temperature in Kelvin scale, is related to temperature in Celsius scale as
(d) Let and be the temperature in Fahrenheit and absolute scale. From the co-rela
New answer posted
8 months agoContributor-Level 10
11.3 It is given that R = [1 + α(T – )] ………(i)
Where Ro and To are the initial resistance and temperature and R and T are the final resistance and temperature.
At the triple point of water, To = 273.15 K, = 101.6 Ω
At normal melting point of lead, T = 600.5 K, R = 165.5 Ω
Substituting these values in equation (i), we get
165.5 = [1 + α(600.5 – )]
= 1 + 327.35 α, or α = = 1.92
When R = 123.4 Ω, T can be calculated as
123.4 = [1 + 1.92 (T – )]
1.214 = 1 + T1.92 - 1.92 273.15
T = 384.6 K
New answer posted
8 months agoContributor-Level 10
11.2 Triple point of water on absolute scale A, = 200 A
Triple point of water on absolute scale B, = 350 B
Triple point of water on absolute Kelvin scale, = 273.15 K
The temperature 273.15 K on Kelvin scale is equivalent to 200 on absolute scale A
200 A = 273.15 K, Therefore A =
Similarly B =
If is the triple point of water on scale A and is the triple point of water on scale B, we have
=
=
New answer posted
8 months agoContributor-Level 10
11.1 Kelvin and Celsius scales are related as
= - 273.15 ….(i),
Where temperature in Celsius scale and = temperature in Kelvin scale
Celsius and Fahrenheit scales are related as
= , where = temperature in Fahrenheit scale
(a) For Neon, = 24.57. Hence = 24.57 – 273.15 = -248.58 degree Celsius
= = -415.44 degree Fahrenheit
(b) For Carbon dioxide, = 216.55. Hence = 216.55 – 273.15 = -56.6 degree Celsius
= = -69.88 degree Fahrenheit
New answer posted
8 months agoContributor-Level 10
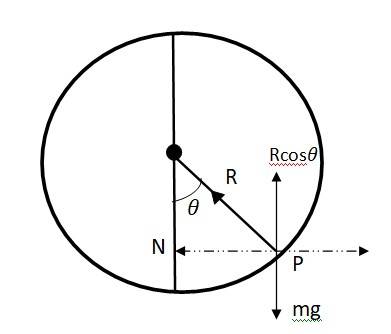
Let be the angle made by the radius vector joining the bead and the centre of the wire with the downward direction. Let, N be the normal reaction.
mg = N …….(1)
mr = N ……(2)
m(R ) = N
Hence N = m(R)
Substituting the value on N in eqn (1)
mg = mR
or = g/ R ………(3)
As 1, the bead will remain at the lowermost point
g/ R
For = becomes
= g/ R
=(g/R)(R/2g) = ½
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
