Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalised n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow cyclic overlap of p-orbitals.
- It should contain Huckel number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3 etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
New answer posted
8 months agoContributor-Level 10
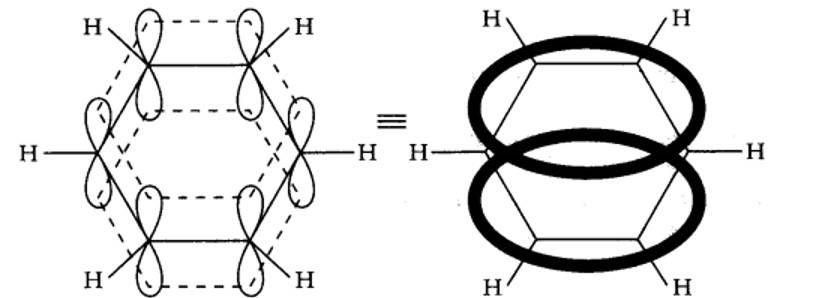
Benzene is a resonance hybrid of two canonical forms. In the resonance hybrid, all the six pi electrons are completely delocalized. This results in resonance stabilization.
New answer posted
8 months agoContributor-Level 10
The structures of cis- and trans-isomer of hex-2-ene are:
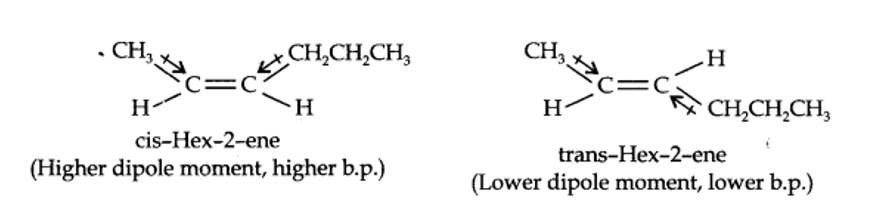
The boiling point of a molecule depends upon dipole-dipole interactions. Since cis-isomer has higher dipole moment, therefore, it has higher boiling point.
New answer posted
8 months agoContributor-Level 10
3.40. Within a period, the oxidising character increases from left to right. Therefore, among F, O and N, oxidising power decreases in the order: F > O > N. However, within a group, oxidising power decreases from top to bottom. Thus, F is a stronger oxidising agent than Cl. Further because O is more electronegative than Cl, therefore, O is a stronger oxidising agent than Cl. Thus, overall decreasing order of oxidising power is: F > O > Cl > N, i.e., option (b) is correct.
New answer posted
8 months agoContributor-Level 10
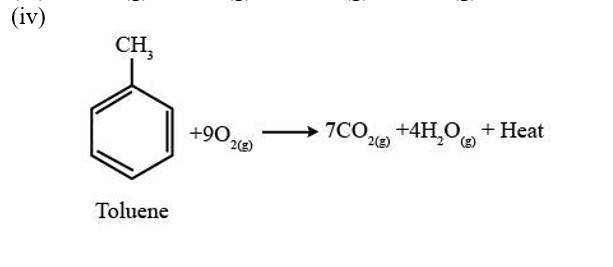
A combustion reaction is a reaction in which a substance reacts with oxygen gas, there is a formation of carbon dioxide, water with the evolution of light and heat.
(i) 2C4H10 (g) +13 O2 (g)?8CO2 (g)+10H2O (g) + Heat
(ii) 2C5H10 (g) +15 O2 (g)?10CO2 (g)+10H2O (g) + Heat
(iii) 2C6H10 (g) +17 O2 (g)?12CO2 (g)+10H2O (g) + Heat
New answer posted
8 months agoContributor-Level 10
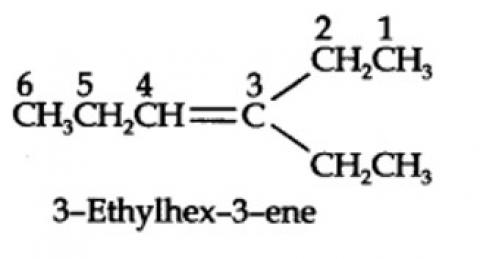
The ozonolysis of 4-Ethylhex-3-ene gives propanal and pentan-3-one.
The structural formula of the alkene (4-Ethylhex-3-ene) is as shown.
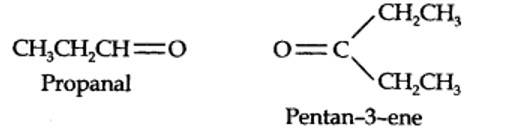
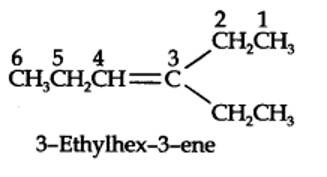
New answer posted
8 months agoContributor-Level 10
3.39. In a period, the non-metallic character increases from left to right. Thus, among B, C, N and F, non-metallic character decreases in the order: F > N > C > B. However, within a group, non-metallic character decreases from top to bottom. Thus, C is more non-metallic than Si. Therefore, the correct sequence of decreasing non-metallic character is: F > N > C > B > Si, i.e., option (c) is correct.
New answer posted
8 months agoContributor-Level 10
(i) An aldehyde with molar mass of 44 u is ethanal, CH3CH=0
(ii) Write two moles of ethanal side by side with their oxygen atoms pointing towards each other.
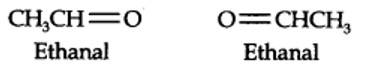
New answer posted
8 months agoContributor-Level 10
3.38. In a period, metallic character decreases as we move from left to right. Therefore, metallic character of K, Mg and Al decreases in the order: K > Mg > Al. However, within a group, the metallic character, increases from top to bottom. Thus, Al is more metallic than B. Therefore, the correct sequence of decreasing metallic character is: K > Mg > Al > B, i.e., option (d) is correct
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
