Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
Excess of CO2 absorbs heat radiated by the earth. Some of it is dissipated into the atmosphere while the remaining part is radiated back to the earth. As a result, temperature of the earth increases. This is the cause of global warming.
New answer posted
8 months agoContributor-Level 10
1.7. 1 mole of CuSO4 contains 1 mole (1 g atom) of Cu
Molar mass of CuSO4= 63.5 + 32 + (4 x 16) = 159.5 g mol-1
Thus, Cu that can be obtained from 159.5 g of CuSO4 = 63.5 g
∴ Cu that can be obtained from 100 g of CuSO4 =63.5/159.5 * 100 = 39.81g
New answer posted
8 months agoContributor-Level 10
The highly poisonous nature of CO arises because of its ability to form a complex with haemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen round the body and ultimately resulting in death.
New answer posted
8 months agoContributor-Level 10
Since, anhydrous HF is a covalent compound and weak acid due to high bond dissociation energy. AlF3does not dissolve in HF.
Whereas NaF is ionic compound.
3NaF + AlF3 → Na3 [AlF6]
Na3 [AlF6] + 3BF3 (g)→ AlF3 + 3Na+ [BF]–
New answer posted
8 months agoContributor-Level 10
B-Cl bond has dipole moment because of polarity. In BCl3, since the molecule is symmetrical (planar), the polarities cancel out and hence the dipole moment is zero
New answer posted
8 months agoContributor-Level 10
1.6. A mass percent of 69% means that 100 g of nitric acid solution contains 69 g of nitric acid by mass.
Molar mass of nitric acid HNO3= 1 + 14 + (3x16) = 63 gmol-1
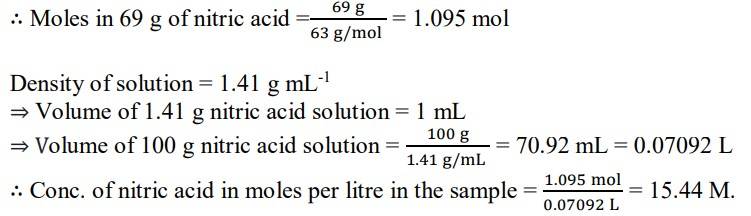
New answer posted
8 months agoContributor-Level 10
The difference in bond length is due to the difference in the state of hybridisation. In BF3 'B' is sp2 hybridised and in BF4– 'B' is sp3 hybridised.
New answer posted
8 months agoContributor-Level 10
1.5
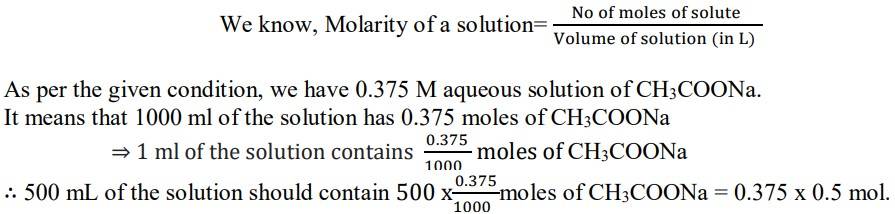
Since the molar mass of sodium acetate is 82.0245 g mol-1.
⇒ 1 mol of CH3COONa has a mass = 82.0245 g
∴Mass of sodium acetate (CH3COONa) required to make 500 ml of 0.375 molar aqueous solution
= 0.375 x 0.5 mol x 82.0245 g mol-1
= 15.3795 g = 15.380 g
New answer posted
8 months agoContributor-Level 10
- PbCl2 + Cl2→ PbCl4.
This is because Pb can show +2 oxidation state more easily than +4 due to inert pair effect. - PbCl4→ PbCl2 + Cl2
Because Pb2+ is more stable than Pb4+ due to inert pair effect. - PbI4does not exist because I- ion being a powerful reducing agent reduces Pb4+ ion to Pb2+ ion in solution.
Pb4+ + 2I– → Pb2+ + I2
New answer posted
8 months agoContributor-Level 10
Diamond has a crystalline lattice where each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three- dimensional network of carbon atoms. It is very difficult to break extended covalent bonding and, therefore, diamond is a the hardest substance on the earth.
Graphite has layered structure in which the layers are held by van there Waals forces and distance between two layers is 340 pm. Each layer is composed of planar hexagonal rings of carbon atoms. C—C bond length
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
