Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
1.17. 15 ppm means 15 parts per million i.e.15 in 106
So, % by mass = 15/106 x 100 = 15 x 10-4 = 1.5 x 10-3%
Molality = No. of moles of solute/Mass of solvent in kg
Percent by mass = 1.5 x 10-3 % means 100 g of the sample contain 1.5 x 10-3 g chloroform.
So, 1000 g or 1 kg of the sample will contain 1.5 x 10-3 x 1000/100 = 1.5 x 10-2 g chloroform.
Molar mass of chloroform = 12 + 1 + (3 x 35.5) = 119.5 g/mol
Therefore, molality = 1.5 x 10-2 /119.5 = 1.26 x 10-4m.
New answer posted
8 months agoContributor-Level 10
1.16. Significant figures are meaningful digits which are known with certainty plus one which is estimated or uncertain. The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures
New answer posted
8 months agoContributor-Level 10
1.15. micro = 10–6, deca = 10, mega = 106, giga = 109, femto = 10–15
New answer posted
8 months agoContributor-Level 10
Hydrolysis of aikyltrichlorosilanes gives cross-linked silicones.
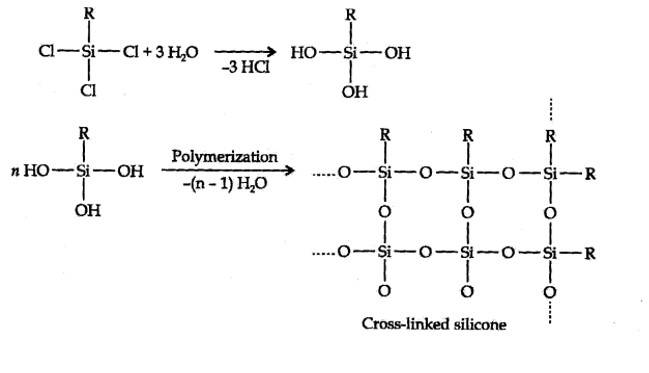
New answer posted
8 months agoContributor-Level 10
Due to inert pair effect, elements of group 14 exhibit oxidation states of +2 and +4. Thus, option (b) is correct.
New answer posted
8 months agoContributor-Level 10
Thermodynamically the most stable form of carbon is graphite, i.e., option (b) is correct.
New answer posted
8 months agoNew answer posted
8 months agoContributor-Level 10
Boric acid is polymeric due to the presence of H-bonds. Therefore, option (b) is correct.
New answer posted
8 months agoContributor-Level 10
Borax is a salt of a strong base (NaOH) and a weak acid (H3BO3), therefore, it is basic in nature, i.e., option (c) is correct.
New answer posted
8 months agoContributor-Level 10
Laboratory preparation of carbon monoxide:
Formic acid is dehydrated with concentrated sulphuric acid at 373 K.
HCOOH → H2O + CO↑
Commercial preparation of CO:
Steam is passed over hot coke.
C + H2O → CO + H2
Laboratory preparation of carbon dioxide:
Calcium carbonate reacts with dilute HCl to form carbon dioxide.
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Industrial preparation of carbon dioxide:
Limestone is heated to produce carbon dioxide.
CaCO3 →→CaO + CO2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
