Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Veronal is a barbiturate drug and it constitutes an important class of tranquilizers.
New answer posted
3 months agoContributor-Level 10
Chloroprene is the monomer of neoprene.
2-chlorobuta-1,3-diene (chloroprene)
New answer posted
3 months agoContributor-Level 10
CH? CONH? - (i) LiAlH? , (ii) H? O? )-> CH? NH?
CH? CONH? - (Br? /KOH)-> CH? NH?
CH? CN - (i) LiAlH? , (ii) H? O? )-> CH? NH?
CH? NC - (i) LiAlH? , (ii) H? O)-> CH? NHCH?
Methyl isocyanide gives a secondary amine, CH? NHCH? upon reduction.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
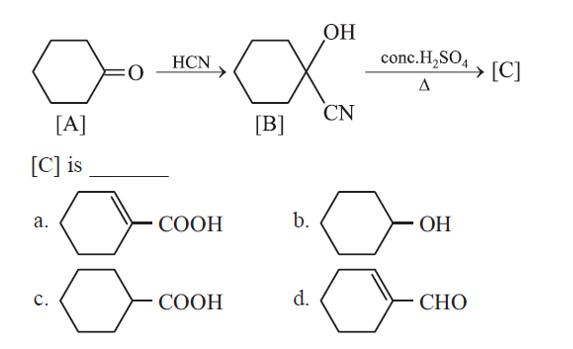
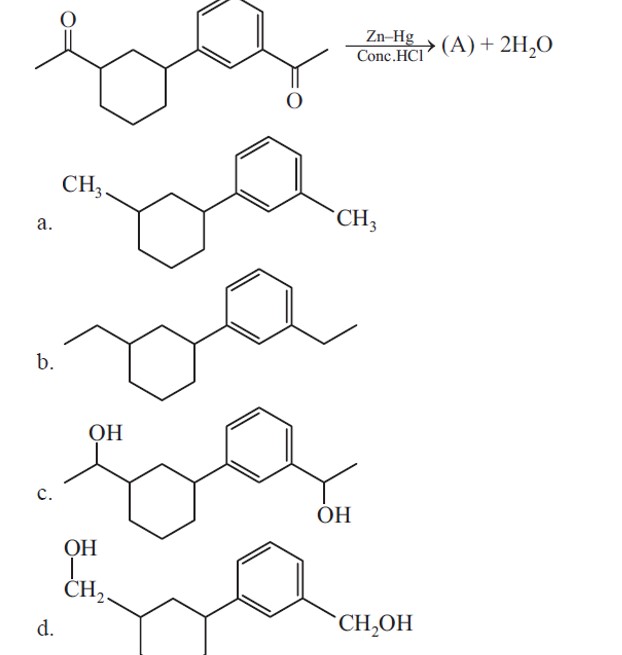
Ketones are reduced into hydrocarbons using Zn – Hg/HCl (Clemmenson reduction).
New answer posted
3 months agoContributor-Level 10
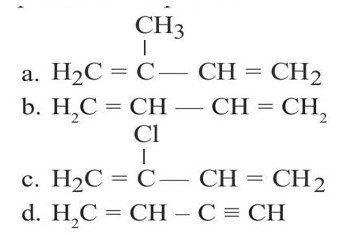
Allylic halide have halogen bonded to sp³ carbon which is adjacent to > C = C <
New answer posted
3 months agoContributor-Level 10
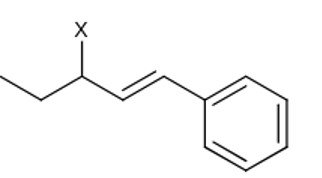
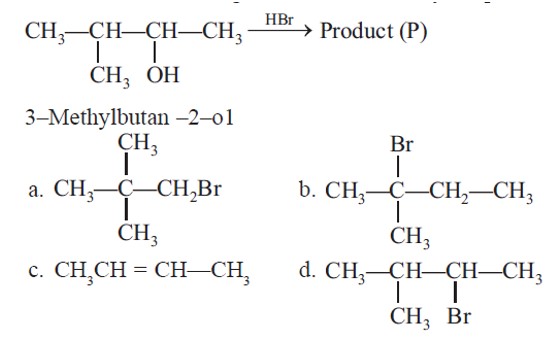
Initially, after protonation followed by loss of water, secondary carbocation is formed, further Hydride shift leads to 3° carbocation.
New answer posted
3 months agoContributor-Level 10
Homoleptic complexes have all ligands identical. Potassium trioxalatoaluminate (III) is K? [Al (C? O? )? ] which has only oxalate ion as ligand. All others have more than one type of ligands.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers