Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Focus of a spherical convex mirror is in the same side of centre of curvature. Thus, f = +
New answer posted
4 months agoContributor-Level 10
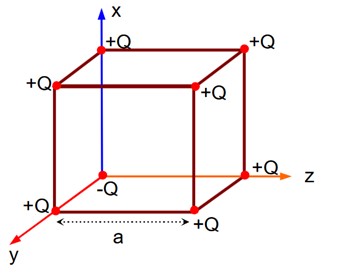
If charge (-Q) at origin is replaced by (+Q), then electric field at the centre of the cube is zero. Thus, electric field at the centre of the cube is as if only (-2Q) charge is present at the origin.
New answer posted
4 months agoContributor-Level 10
Moles of SO2 =
=0.01 mole
Moles of NaOH = 0.1 * 0.1
= 0.01 mole
SO2+NaOHNaHSO3
0.01 mole0.01 mole-
-0.01 mole
Non-volatile solute is NaHSO3
Moles of water =
Using ; relative lowering in V.P
Where; is lowering in V.P
i for NaHSO3 = 2
here; since solution is dilute
So; x = 24
New answer posted
4 months agoContributor-Level 10
PV = nRT
1 * V =
V = 2.4 litre
Vol of O2 adsorbed per gm = 2.4 / 1.2 = 2 litre
New answer posted
4 months agoDichromate ion is treated with base, the oxidation number of Cr in the product formed is-----------.
Contributor-Level 10
Dichromate ion converted to chromate ion in basic medium and oxidation number of Cr in is +6.
New answer posted
4 months agoContributor-Level 10
Quantity of electricity required to reduce 1 mole of is 5F
So, for 5 mole 25F electricity is required.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers