Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
When red phosphorus is heated in a sealed tube at 803 K, a - black phosphorus is formed.
New answer posted
5 months agoContributor-Level 10
t = 00.8 M
0 5 * 10-8 M 0.8M
Concentration of NH3 added =
Volume of solution = 2L
Moles of NH3 added = 2 * 2 mol = 4 mol.
New answer posted
5 months agoContributor-Level 9
E = (50 N/s) sin
Energy 5.5 * 10-12 J., vol v =?
energy density
=
= 497
New answer posted
5 months agoNew answer posted
5 months agoContributor-Level 10
Let molarity of KMnO4 = x
n = 5 n = 1 Ferric sulphate
equivalent of KMnO4 = equivalent of FeSO4
5 * x * 10 = 1 * 0.1 * 10
x = 0.02 M
Strength = (0.02 * 158) = 3.16g/L
= 316 * 10-2 g/L
Ans. = 316
New answer posted
5 months agoContributor-Level 10
Dumas method,
Moles of N in N, N-dimethylaminopentane (C7H17N)
= 11.25 mol
= 1125 * 10-2 mol
Ans. = 1125
New answer posted
5 months agoContributor-Level 10
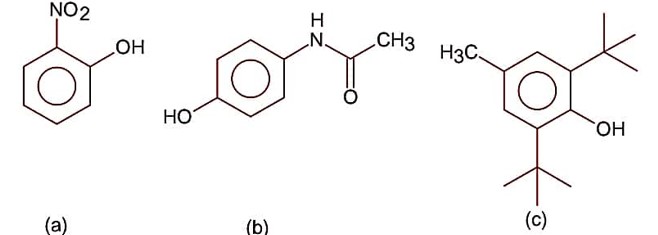
(a) Shows intra molecular H-bonding.
(b) Shows inter molecular H-bonding.
(c) It does not shows intermolecular H-bonding due to high steric hindrance at o-position of benzene ring.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers