Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
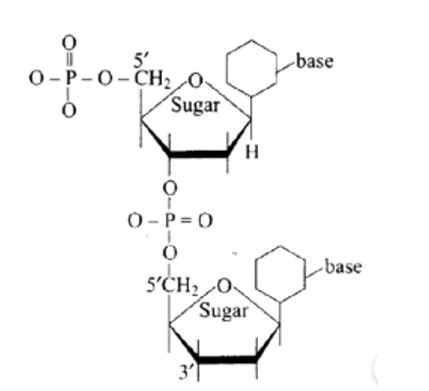
(a) 5′ and 3′ linkages are present between pentose sugars of nucleotides.
Phosphodiester links the two nucleotides together to form a dinucleotide.
Between the pentose sugars of nucleotides, there are 5′ and 3′ connections. In the process of adding phosphate to a dinucleotide, a significant quantity of energy is consumed.
New answer posted
7 months agoContributor-Level 10
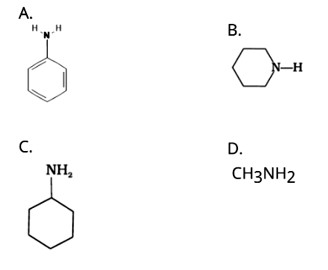
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A)
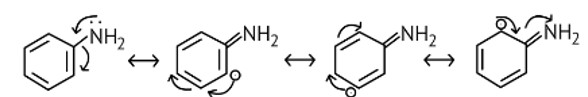
The NH2 group is directly attached to the benzene ring in aniline and other aryl amines. As a result, the unshared electron pair on the nitrogen atom is conjugated with the benzene ring, making it less available for protonation.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(d) All soaps are made by boiling fats or oils with suitable hydroxide. Variations are made by adding different raw materials. Sodium laurylsulphate and sodium dodecylbenzenesulphonate are anionic detergents.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Ans: The Correct option is b.
Ascorbic acid is the chemical name for vitamin C. Aspartic acid is an amino acid, adipic acid is a dicarboxylic acid with an eight-carbon chain, and saccharic acid is a dicarboxylic acid made by oxidising glucose with HNO3 Vitamin C is a water-soluble vitamin that serves a variety of activities in the human body.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
Because of the electron-releasing nature of the alkyl group, it (R) pushes electrons towards nitrogen, making the unshared electron pair more available for sharing with the acid's proton. Furthermore, the +I effect of the alkyl group stabilizes the substituted ammonium ion formed from the amine by dispersing the positive charge.
As a result, alkylamines are more powerful bases than ammonia.
As a result, the basic nature of aliphatic amines should increase as the number of alkyl groups increases.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct answer: (D)
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) The compound that causes general antidepressant action on the central nervous system belongs to the class of tranquilizers.
New answer posted
7 months agoContributor-Level 10
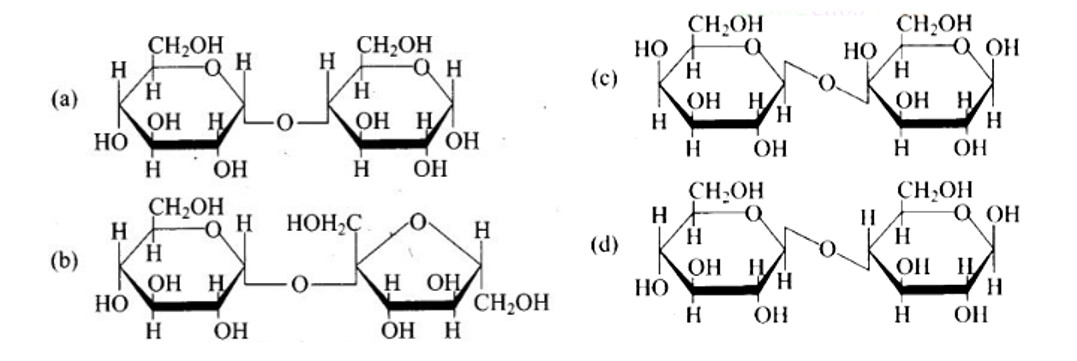
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Ans: The Correct option is b.
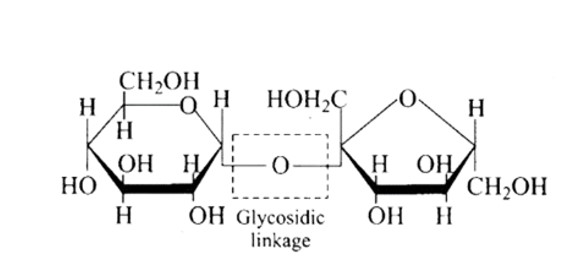
The structure described in option (b) reflects sucrose, which has a C1−C2 glycosidic bond between α−D− glucose and β−D− fructose.
This is a non-reducing sugar since the reducing groups of glucose and fructose are involved in the creation of glycosidic linkages.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
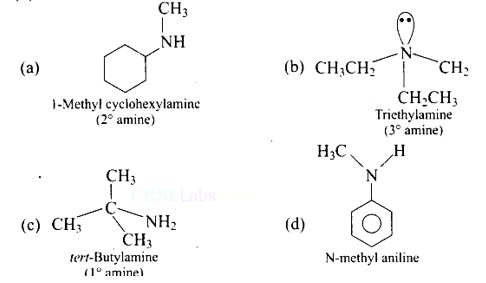
Ans: (B) Triethylamine
The structure of the given amines are shown below.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a) A narrow spectrum antibiotic is active against gram positive or gram negative bacteria.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers