Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
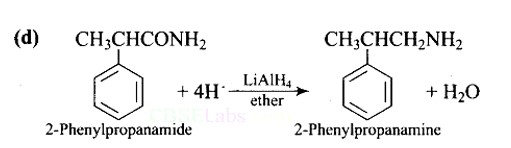
Lithium aluminium hydride in ether is a strong reducing agent that donates its hydride ion to any C=O containing functional groups into corresponding reduced compounds. Here, the amide group is converted to an amine functional group.
LiAlH4 in ether is the best reagent for converting 2-phenylpropanamide to 2-phenylpropanamine.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) Shaving soaps contain glycerol to prevent rapid drying. A gum called rosin is added in these soaps which forms sodium rosinate which enhances lathering property of soap.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: ( C)
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Gabriel synthesis reaction:
Gabriel's synthesis is a method for producing primary amines. When phthalimide is treated with ethanolic potassium hydroxide, it forms a potassium salt of phthalimide, which when heated with an alkyl halide and then alkaline hydrolyzed yields the corresponding primary amine. This method cannot produce aromatic primary amines because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
Potassium cyanide, as cyanide on reduction with sodium metal in alcohol, produces amine with increased carbon atoms.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Lithium aluminum hydride in ether is a strong reducing agent that donates its H? hydride ion to any C=O containing a functional group. In addition to LiAlH4 to aryl nitro compounds, no reaction will be observed, the desired products of amines will not be produced, rather it will form diazobenzene products.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
SN1 reaction: A nucleophilic reaction that occurs in two steps, first is the bond-breaking step and the second is the production of the carbocation. The stability of carbocation formed in the second step determines the rate of reactivity of reactant toward SN1 reaction. Here, C6H5CH2Br,
In the process of ionization, removal of bromine, a stable Benzyl carbocation is produced. Therefore, it is best suited for reaction through the SN1 mechanism.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
The pyranose form of glucose, which has a five-membered ring structure, is found. As a result, option (c) is wrong.
Glucose is a six-carbon monosaccharide with an aldehyde group. As a result, it's referred to as an aldohexose. As a result, the statement in option (a) is correct.
When glucose is cooked with HI, n-hexane is produced. All six carbon atoms in glucose are linked together in a straight chain during this procedure. As a result, the statement in option (b) is correct.
The aldehyde group is not free in the cyclic structure. As a result,
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) Equanil is a tranquilizer used in controlling depression and hypertension.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is b.
Nucleic acids are nucleotide polymers linked together by phosphodiester linkage. DNA and RNA are two examples. Nucleotides are formed by linking of nucleosides with three phosphate groups consuming a significant amount of energy making it an endothermic reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers