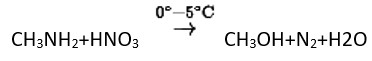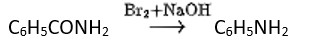Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(d) Sucralose is an artificial sweetening agent which is 600 times sweeter than sucrose and does not provide calories.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Inhibitors are chemical substances which tend to reduce the activity of a particular enzyme. Generally, a weak bond such as H-bonding, van there Waals interaction, etc. is formed between the enzyme and the inhibitor.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
Methyl amine reacts with HNO3 form methanol with release of nitrogen gas and water as side products.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Drugs usually interact with biomolecules such as carbohydrates, lipids, proteins and nucleic acids. These are called drug targets. Vitamins are not a target molecule for drug function in body.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
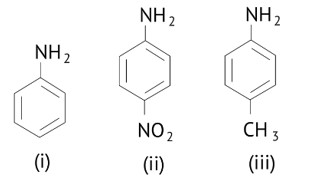
Ans: (D)
The greater the electron density towards the ring, the greater its basic strength.
The electron withdrawing group reduces basic strength, whereas the electron donating group increases basic strength.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Polyethyleneglycols are used in the preparation of non-ionic detergents.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Hoffmann invented a method for producing primary amines by treating an amide with bromine in an aqueous or ethanolic sodium hydroxide solution. An alkyl or aryl group migrates from the amide's carbonyl carbon to the nitrogen atom during this degradation reaction.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) Liquid dishwashing detergents are non-ionic detergents.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Hoffmann bromamide reaction- it is also called degradation reaction as in this reaction primary amide group is treated with halogen first (Br) then the halogen-substituted amide product is converted to a primary amine with the release of carbon dioxide gas.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Glycerol is added to shaving soap to prevent rapid drying while to enhance the leathering property of soap, a gum called rosin is added to them. It forms sodium rosinate which lathers well.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers