Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A)
The basic strength of an atom is determined by its electron-donating capacity; in this case, the amide is the most basic due to the presence of a negative charge and two lone pairs of electrons on the nitrogen atom.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
The absence of a free (-CHO) aldehyde group is indicated by the fact that glucose pentaacetate does not react with hydroxylamine. The open structure of glucose cannot account for this, although the open chain structure of glucose can account for all other features.
New answer posted
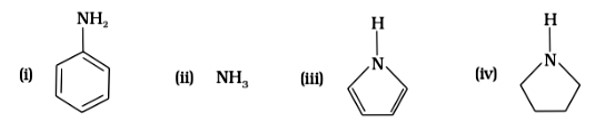
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Pyrrolidine is the strongest of two bases because the lone pair of nitrogen does not involve sin resonance, and the presence of two alkyl basic compounds increases the basic strength among the given four compounds.
New answer posted
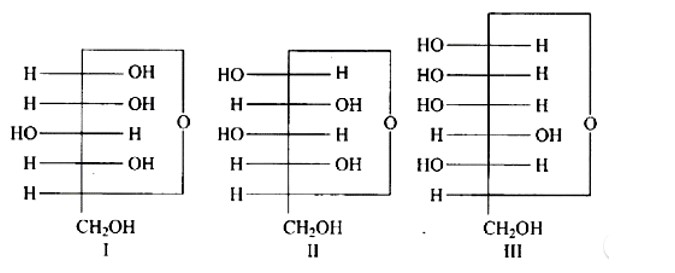
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
Ans: The Correct option is a.
Anomers are cyclic monosaccharide structures that differ structurally at carbon-1. In this scenario, I and II are anomers since they differ only at carbon -1.
New answer posted
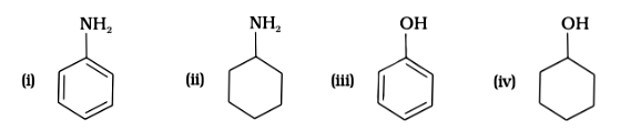
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Since phenol is the strongest acid among the four options listed above, it is the weakest Brönsted base. The stronger the acid, the weaker the conjugate base.
Amines have a strong tendency to accept electrons thus they are a strong bronsted base while phenol is the strongest acid among all, therefore as per the relation of conjugative strong acids and weak bases, phenol is the weakest base.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is d.
Adenine, guanine, thymine, and cytosine are the four bases found in DNA. As a result, uracil is not found in DNA as we cannot identify it among the other nucleotides that are present in the DNA. Uracil is, however, seen in RNA, another nucleic acid found in most of the organisms.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Weak electrophile such as Diazonium cation readily reacts with electron-rich compounds which are having electron-rich compounds such as hydroxyl group, amino group. They don't react with electron-withdrawing groups like nitro groups. Therefore, nitrobenzene will not undergo an azo coupling reaction with benzene diazonium chloride.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is d.
Water-soluble vitamins are those in the B group. B group vitamins cannot be stored in our systems since they are quickly excreted; but, because they are B12 insoluble in water, our bodies may store the vitamin. Because our systems are unable to store these vitamins, options (a), (b), and (c) are excluded.
New answer posted
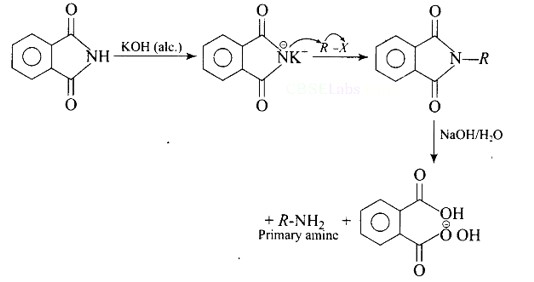
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
The reaction of transformation of primary alkyl halides to primary amines using potassium phthalimide is the Gabriel phthalimide reaction.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
The four bases contained in DNA are adenine, guanine, thymine, and cytosine. Adenine, uracil, guanine, and cytosine are the four bases of RNA. As a result, RNA does not include thymine as in place of thymine, it has uracil which is a lesser stable nucleotide base and is somewhat responsible for RNA's instability but also lends it some flexibility to change.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers