Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
The difference between electrode potential potentials of two electrodes when no current is drawn through the cell is termed as cell emf. It is the energy provided by a cell per coulomb of charge passing through it. It is the maximum potential difference between the electrodes of a cell.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
At room temperature, the redox titration of KMnO4vs oxalic acid is exceedingly slow; nevertheless, as the temperature rises, so does the rate of reaction. At a temperature of 500c., KMnO4 is acidified with dil H2SO4 and interacts with oxalic acid.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
Thermodynamic feasibility is determined by ΔG factors. So, while the conversion of diamond to graphite is thermodynamically viable, the reaction is sluggish and has a high activation energy.
Gibb's free energy is ΔG
ΔG =Rtlnk
ΔGP, T o
at constant pressure and temperature should be negative or less than 0.
For example, Diamond to be converted to Graphite
ΔG=−ve
It is theoretically conceivable, but the rate is extremely slow, and the activation energy is extremely high. The energy required to transform diamond to graphite is insufficient.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i) The properties which don't depend on the mass of species are termed intensive properties. ECell is an intensive property that doesn't depend on the mass of the species.? rG is an extensive property as it depends on the mass of the species. Therefore, this option is incorrect
(ii) ECell is an intensive property but? rG is an extensive property so this option is incorrect
(iii) The properties which don't depend on the mass of species are termed intensive properties ECell is an intensive property that doesn't depend on the mass of the species
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
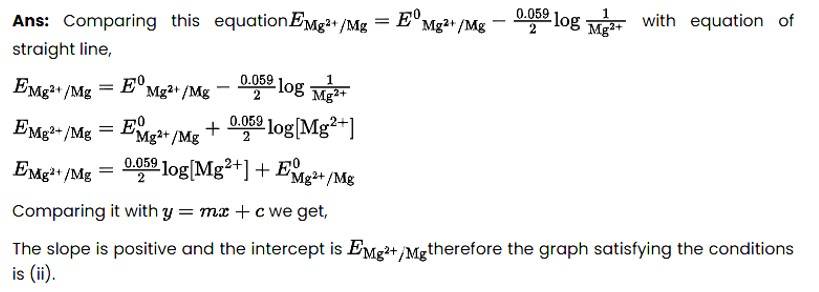
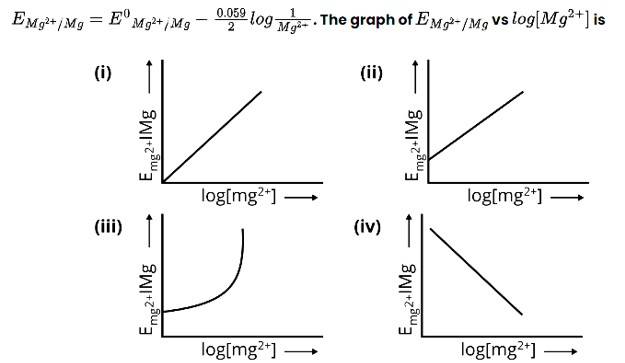
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The pace of any reaction is determined by the concentration of the reactants, the rate of any reaction generally declines during the reaction. As the reaction progresses, the reactant concentration declines, and the product concentration rises.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i) For measurement of electrode potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(ii) For measurement of electrodes potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(iii) When copper electrode is connected to SHE it acts as cathode and its standard electrode potential can be measured as follows
E E R- E L
For calculationn of standard potential of a given cell it shoul
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Acetic acid is a weak electrolyte and the number of ions on dilution increases due to an increase in the degree of dissociation. In the case of strong electrolytes the number of ions remains the same but interionic attraction decreases.
New answer posted
7 months agoContributor-Level 10
Xenon form compounds due to the high electronegativity of fluorine and oxygen, low ionization energy compared to lighter noble gases, and the availability of empty d-orbitals. This breaks the old assumption about noble gases being entirely inert.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The cell reaction of a lead storage battery is as follows;
Pb + PbO2 + 2H2SO4→2H2SO4 + 2H2O
The density of electrolytes changes because water is formed and sulphuric acid is consumed as a product during discharge of the battery
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers