Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
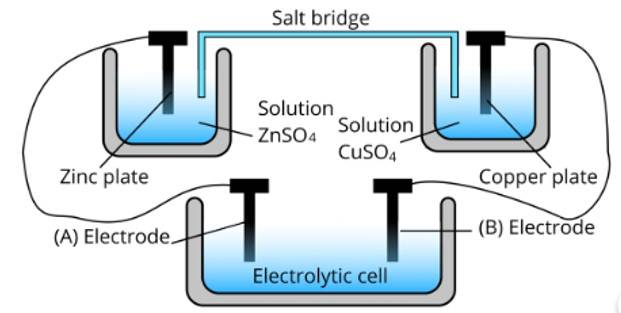
Ans: (i) Cell 'B' will act as an electrolytic cell because to less value of Ecell The reactions occurring in the cell are as follows;
At anode: Zn2+ + 2e− → Zn
At cathode: Cu (s) → Cu2+ + 2e−
(ii) Cell 'B' has a higher emf so it acts as a galvanic cell. The reactions are as follows;
At anode: Zn → Zn2+ + 2e−
At cathode: Cu2+ + 2e− → Cu
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Average rate depends upon the change in concentration of reactants or products and the time taken for that change to occur. However, the average rate cannot be used to predict the rate of reaction at a particular instance as it would be constant for the time interval for which it is calculated.
So, to express the rate at a particular moment of time we determine the instantaneous rate. Itis obtained when we consider the average rate at the smallest time interval say dt (i.e. when? t approaches zero).
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Ans: Catalyst action is a complicated concept. A catalyst aids in the formation of temporary bonds during a chemical reaction. The objective of a catalyst is to lowers the activation energy. A catalyst is a substance that increases the rate of a reaction without itself undergoing any permanent chemical change. The action of the catalyst can be explained by intermediate complex theory. According to this theory, a catalyst participates in a chemical reaction by forming temporary bonds with the reactants resulting in an intermediate complex. This has transitory existe
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Ans:
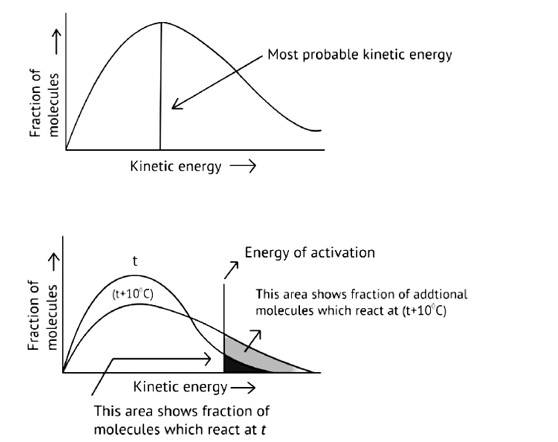
As illustrated in the graph, as the temperature rises, the peak pushes ahead, increasing probable kinetic energy while decreasing the number of molecules utilizing it, resulting in a faster rate of reaction.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
The reaction rate in collision theory is determined by two factors: energy and orientation factor. Certain reactions can be highly exothermic and energetically favoured, meaning the reactants have enough activation energy to collide effectively. However, they do not proceed at a fixed temperature, which is why the reactant molecules are not orientated properly during collisions, resulting in atoms of reactant molecules combining to produce products that do not face each other.
New answer posted
7 months agoContributor-Level 10
This is an Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is an Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is an Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is an Objective Type Questions as classified in NCERT Exemplar
Sol:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers