Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
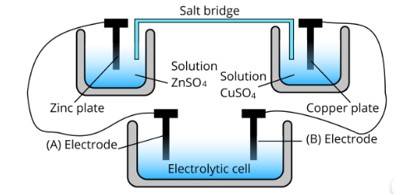
Ans: The cell represented above shows an electrochemical cell in which two different electrodes are present and the cell at the bottom represents the electrolytic cell.
In this zinc is losing electrons moving towards electrode A and copper is accepting an electron from electrode B. Therefore, the polarity of electrode A is positive and electrode B is negative.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
When the Threshold energy is less than the energy of the molecules and the orientation of the molecules is proper for the effective collision, the reaction happens. As a result, the reaction is slow, and the number of effective collisions is reduced
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The potential difference between the metal and its solution is termed electrode potential.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The number of ions or atoms that collide to react is known as molecularity in an elementary reaction. The order of reaction about B should be 1 if this is an elementary reaction, however the rate law given is
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In the electrolysis process of sodium chloride, oxidation of water at anode requires more potential. So Cl− is oxidized at anode instead of water.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The concentration of the reactants does not change with time in a zero- order reaction, and the rate of concentration remains constant.
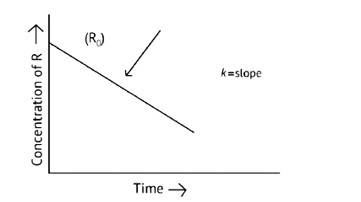
R= [R]0 – Kt
Kt = [R]0
t =
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Galvanic cell consists of two electrodes in which anode and cathode are present as electrodes. The anode is present on the left side at which oxidation occurs. The cathode is present on the right side at which reduction occurs and in the middle, there is a salt bridge which is depicted by parallel lines. Therefore, the galvanic cell is Cu|Cu2+ |Ag+ |Ag.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: According to Faraday's second law of electrolysis, the amount of different substances liberated keeping the electricity flow same through the electrolytic solution is directly proportional to the equivalent weight.
=
E1and E2 have different values. Therefore, the mass of copper and silver deposited will be different
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The concentration of the reactants does not change with time in a zero- order reaction, and the rate of concentration remains constant.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: At the stage of equilibrium E? Cell =0. So according to the relation,
ΔrG =−nFEcell
ΔrG =−n*F*0
ΔrG =0
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers