Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
Let order of reaction be = n
rate = k [A]n- (i)
27r = k [3A]n- (ii)
Divide (ii) by (i)
= k [3A]nn
3n = 27
n = 3
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: E? Cell can never be zero. For a feasible reaction E? Cell should be positive or ΔrGshould be negative and at the stage of equilibrium, both of these parameters are zero.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The order and molecularity of elementary reactions are the same. As a result of a single collision between two molecules or ions, a complicated process occurs. A reaction mechanism is a set of elementary reactions.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:No, the difference in potentials of the electrodes is measured. A reference electrode is to be taken while measuring the electrode potential of the electrode.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
Experimentally, the reaction's rate law can be calculated.
Step 1
2NO (g) + O2 (g)→2NO2 (g)
O2 (g) is taken in excess
x molecules of NO number reacting with excess of O2
r = k [NO]x
Step 2
2NO (g) + O2 (g)→2NO2 (g)
When 2No (g)is taken in excess
Y molecules of O2 reacting with excess of [NO]
rate = k [O2]y
rate = k [NO]x [NO]y
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The relation between Gibbs free energy and the emf of the cell is as follows;
ΔG=−nFEcell
E cell s the cell potential
is the standard emf of the cell
Maximum work obtained from the galvanic cell is nFE .
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
Given: Order of the reaction = 0
We know that a zero order reaction is a chemical reaction that occurs regardless of the reactant's concentration.
The rate law of 2A + B→C is
r = k [A]0 [B]0
For a zero- order reaction
r = k
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (i) The diagram is as follows;
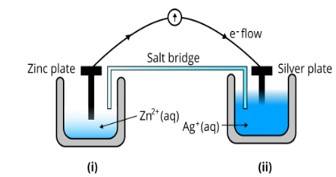
(ii) Agis cathode where the reduction process is taking place where Ag+ takes electrons and deposits them at the cathode
(iii) Potential is zero when the salt bridge is suddenly removed.
(iv) Cell will stop functioning at discharging position when the cell potential is zero
(v) The concentration of Zn2+ ions will increase and the concentration of Ag+ ions will decrease due to conversion in oxidized and reduced forms.
(vi) When the cell is dead, the potential is zero and at equilibrium condition. Thus, the concentration of Zn2+ and A
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
A bimolecular reaction occurs when two particles collide. The product of the concentrations of both elements determines the rate of reaction. If one of the reactants is taken in substantial excess in such a way that its concentration seldom changes, a bimolecular reaction can be kinetically first order.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Question as classified in NCERT Exemplar
Tell us about the pseudo-first-order reaction. It's the reaction with the highest true rate law, yet it behaves like a first order reaction, but it's more specifically a second order reaction.
As an example, consider the hydrolysis of an ester.
CH3COOC2CH5 + H2O→CH3COOH + C2H5OH
rate = k [CH3COOC2H5] [H2O]… (constant)
k1 [CH3COOC2H5]
k = k [H2O]
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers