Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Conductivity due to the total number of ions present in the solution is termed as specific conductivity. An increase in the number of ions per unit volume increases. In addition to water, the number of ions per unit volume decreases therefore conductivity decreases.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
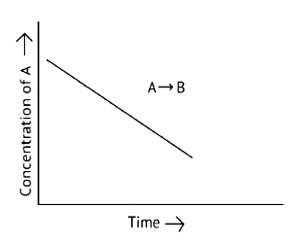
A. The reaction has a zero order. The concentration of the reactants does not change with time in a zero order reaction, and the rate of concentration remains constant
B. Slope of the curve is - k
C. The rate constant's units are molL 1s - 1
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: pH of the substance depends on the concentration of hydronium ions present. Here the concentration of hydronium ions remains constant so pH remains constant.
The electrolysis reaction is as follows;
At anode: 2H2O (l)→O2 (g)+ 4H+ + 4e−
At cathode:4H+ + 4e− →2H2
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Stronger electrolytes undergo cent percent dissociation. Electrolyte B will be the stronger electrolyte since the concentration of ions remains the same upon dilution.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The number of ions present in the cell decides the life-time of any cell. In mercury cells, ions are not involved so the mercury cell has a constant cell potential throughout its useful life.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Electrolysis of brine solution is as follows
NaCl (aq)→Na+ (aq)+Cl− (aq)
At the cathode, the reaction goes this way,
H2O (l) + e−→12H2 (g)+OH− (aq)
At the anode, the reaction goes this way,
Cl− (aq)→12Cl2 (g) + e−
The pH of the solution will increase as sodium hydroxide is being formed.
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: When the opposing potential becomes equal to the electrical potential there is no current flowing in the cell and the cell reaction stops and there is no chemical reaction in the cell
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The concentration of the reactants does not change with time in a zero -order reaction, and the rate of concentration remains constant. The molecularity of a reaction cannot be 0 because it indicates the number of reactants involved.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Alternating current is used to stop electrolysis so that the concentration of the ions in the solution remains constant.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers