Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
8 months agoNew question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
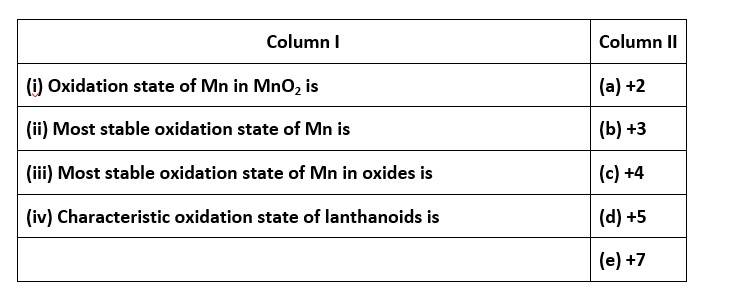
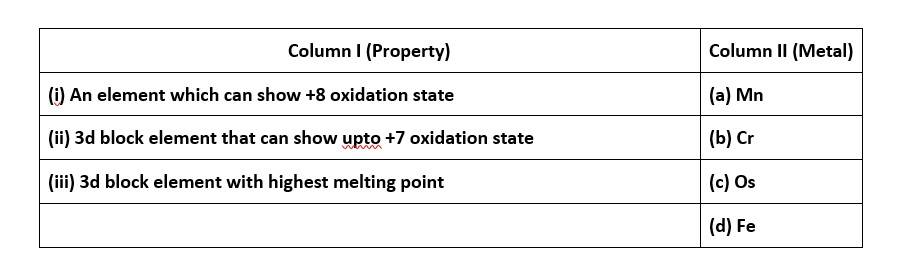
64. Osmium has the capacity to expands its octet by utilising all the electrons from its 6s and 5d orbitals this results it to attain an oxidation state of +8.
Both the statements assertion and reason are correct but reason is not the correct explanation of assertion. The correct option is B.
New answer posted
8 months agoContributor-Level 10
63. Cu lies below hydrogen in electrochemical series and has positive electrode potential. hence cannot liberate hydrogen from acids.
Both the statements assertion and reason are correct and reason is the correct explanation of assertion.
New answer posted
8 months agoContributor-Level 10
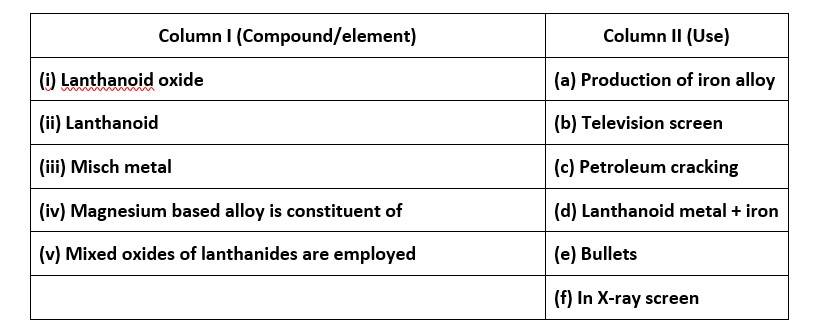
62. Actinoids are more reactive than lanthanoids as they involve their 5d-orbital in the bond formation process while due to effectively shielded and greater nuclear charge 4f-orbitals are not available for bonding. Thus, lanthanoids comparatively form less complexes than actinoids.
Assertion is not true but reason is true.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers