General Principles & Processes of Isolating Elemen
Get insights from 111 questions on General Principles & Processes of Isolating Elemen, answered by students, alumni, and experts. You may also ask and answer any question you like about General Principles & Processes of Isolating Elemen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
According to Ellingham diagram, the metal oxide with lower is more stable than metal oxide of higher at same temperature.
New answer posted
3 months agoContributor-Level 9
Below 1350° C, Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO (from Ellingham diagram).
Melting and boiling point of Mg are lower than that of Al.
New answer posted
4 months agoContributor-Level 10
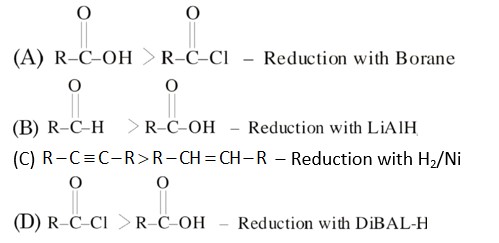
DiBAL-H is electron deficient reducing agent as Burane and readily reduces electron rich sp.
New answer posted
4 months agoContributor-Level 10
The slag formed is CaSIO3 which is lighter and has less melting point.
New answer posted
4 months agoContributor-Level 10
Sphalerite is ore of zinc consists of ZnS during its concentration group 1 cyanides are used as depressants like NaCN
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers