General Principles & Processes of Isolating Elemen
Get insights from 111 questions on General Principles & Processes of Isolating Elemen, answered by students, alumni, and experts. You may also ask and answer any question you like about General Principles & Processes of Isolating Elemen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
(H? S + dil HCl) is 2nd group reagent so Cu²? get precipitated as CuS.
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Reduction half reaction.
Cr? O? ²? + 14H? + 6e? → 2Cr? ³ + 7H? O
Oxidation half reaction
SO? ²? + H? O → SO? ²? + 2e? ] * 3
Oxygen is balanced by adding water and hydrogen is balanced by adding H? and the charge is balanced by electrons.
Add ( eq. (i) + (3 * eq. (ii) )
Cr? O? ²? + 3SO? ²? + 8H? → 2Cr? ³ + 3SO? ²? + 4H? O
a = 1 b = 3 c = 8
New answer posted
4 months agoContributor-Level 10
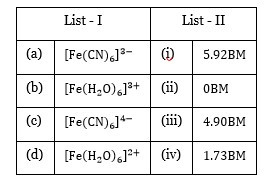
[Fe (CN)? ]? ³ Fe? ³ = 3d?
Unpaired electron = 1, μ = 1.7BM
[Fe (H? O)? ]? ³ Fe? ³ = 3d?
Unpaired electrons = 5, μ = 5.9BM
[Fe (CN)? ]? Fe? ² = 3d?
Unpaired electron = 0, μ = 0BM
[Fe (H? O)? ]? ² Fe? ² = 3d?
Unpaired electrons = 4, μ = 4.9BM
New answer posted
4 months agoContributor-Level 10
Hematite: Fe? O?
Magnetite: Fe? O?
Calamine: ZnCO?
Kaolinite: [Al? (OH)? Si? O? ]
New answer posted
4 months agoContributor-Level 9
Roasting is a process in which sulphur is removed as SO? gas from sulphide ores on heating in excess of oxygen.
New answer posted
4 months agoContributor-Level 10
The reduction of aluminum oxide (Al? O? ) is performed through an electrolytic process in its molten state. This method is necessary because Al? O? is a highly ionic and stable compound.
New answer posted
4 months agoContributor-Level 9
The following depicts a chemical reaction leading to a Major Product Y.
Mercury has low boiling point so is refined by distillation method.
Copper refining is done through electrolytic refining.
Silicon is refined by zone refining method.
Nickel is refined by vapour phase refining.
New answer posted
4 months agoContributor-Level 9
When we add cryolite (Na? AlF? ) in the extraction of aluminium, the melting point of alumina decreases
New answer posted
4 months agoContributor-Level 10
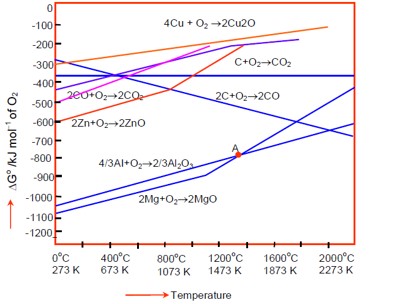
At the point of intersection in an Ellingham diagram? G for two processes becomes equal, so? G for the reduction becomes zero. A sudden increase in the slope indicates a change in the state of the metal oxide, i.e., from solid to liquid or liquid to vapor.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers