General Principles & Processes of Isolating Elemen
Get insights from 111 questions on General Principles & Processes of Isolating Elemen, answered by students, alumni, and experts. You may also ask and answer any question you like about General Principles & Processes of Isolating Elemen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
New answer posted
3 months agoContributor-Level 10
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
New answer posted
3 months agoContributor-Level 10
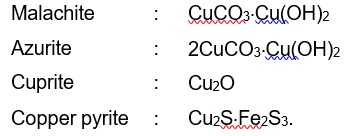
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
New answer posted
3 months agoContributor-Level 10
Aluminium - Kaolinite
Iron - Siderite
Copper - Malachite
Zinc &
New answer posted
3 months agoContributor-Level 10
Sphalerite is ore of zinc consists of ZnS during its concentration group 1 cyanides are used as depressants like NaCN
New answer posted
3 months agoContributor-Level 10
(a) Na2CO3 -> Solvay
(b) Ti -> Van-Arkel
(c) Cl2 -> Deacon
(d) NaOH -> Castner – kellner
New answer posted
3 months agoContributor-Level 10
Siderite – FeCO3 (ore of iron)
Calamine – ZnCO3 (ore of zinc)
Malachite – CuCO3.Cu (OH)2 (ore of copper)
Cryolite – Na3AlF6 (ore of aluminium)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers