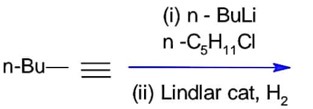Haloalkanes and Haloarenes
Get insights from 279 questions on Haloalkanes and Haloarenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Haloalkanes and Haloarenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
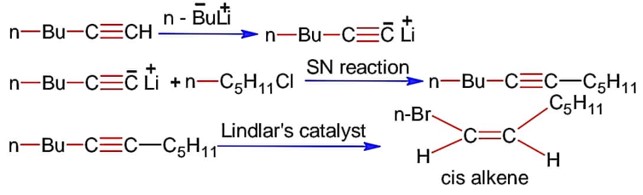
Lindlar's catalyst reduces alkynes to cis-alkene.
Lindlar's catalyst reduces alkynes to cis-alkene.
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Because m-nitrochlorobenzene is not a stable molecule, the -NO2 group is a meta-directing group, and the reactions' products include nitro groups at the o- and p- positions.
Correct Answer: Option (iv)
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
The SN2 mechanism is demonstrated by the hydrolysis of alkyl halides with inversion of configuration. This is a one-step method that does not need the production of carbocation.
Correct Answer: Option (iii)
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Due to resonance, a partial double bond character occurs in the bond between the C and Cl atoms in chlorobenzene, and we all know that changing a partial double bond character is more difficult than replacing a single bond as in the C - Cl bond in chloroethane.
Correct Answer: Option (i)
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Oxidising chemicals such as HIO3 oxidise HI to I2 because the presence of HI causes the aryl iodides to revert to arenes in their absence.
Correct Answer: Option (iii)
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
Because halogen atoms are ortho and para directing, rather than ring deactivators, further electrophilic substitution occurs at ortho and para locations.
Correct Answer: Option (v)
New answer posted
7 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
The nitro group on the ortho and para positions of haloarenes acts as an electron-withdrawing group, making it more reactive to nucleophilic substitution reactions due to the electron deficit.
Correct Answer: Option (i)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers