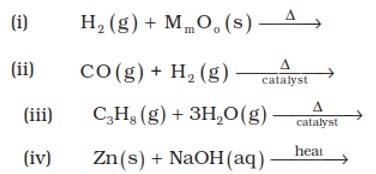Hydrogen
Get insights from 182 questions on Hydrogen, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrogen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
9.2.

Mass ratio of the isotopes = Protium: Deuterium: Tritium = 1: 2 :3
New answer posted
9 months agoContributor-Level 10
9.1. Hydrogen has electronic configuration 1s1similar to the outer electronic configuration (ns1) of alkali metals, which belong to the first group of the periodic table. On the other hand, like halogens (with ns2np5) configuration belonging to the seventeenth group of the periodic table, it is short by one electron to the corresponding noble gas configuration, helium (1s2). Hydrogen, therefore, has a resemblance to both alkali metals, which lose one electron to form unipositive ions, as well as with halogens, which gain one electron to form uni-negative ion. Like alkali metals, hydrogen forms oxides, halides and sulphides. Howeve
New answer posted
9 months agoContributor-Level 10
Answer: (i) 3H2? (g)+2MoO3? ? Mo2? O3? +3H2? O (l)
(ii) CO (g) + H2 (g)? CH3OH
(iii)C3H8 (g) + 3H2O (g)? 3CO + 7H2 (g)
(iv) Zn (s) + NaOH (aq)? Na2ZnO2 (s) + H2 (g)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers