Hydrogen
Get insights from 182 questions on Hydrogen, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrogen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
(i) Industrial preparation: H2O2 is prepared by the auto-oxidation of 2- alkylanthraquinols
2-ethylanthraquinol ↔ H2O2+ oxidised product
2Fe2+ (aq)+2H+ (aq)+H2O2 → Fe3 (aq)+2H2O (l)
PbS (s)+4H2O2 (l) → PbSO4 (s)+4H2O (l)
(ii) Reducing action of hydrogen peroxide
2MnO4-+6H++5H2O2→ 2Mn2++8H2O+5H2
HOCl+H2O2 → H3O++Cl-+O2
Oxidising action of hydrogen peroxide
2Fe2++H2O2→ 2Fe3++2OH-
Mn2++H2O2 → Mn4++2OH-
Acidic properties of H2O2
I2+H2O2+2OH-→ 2I-+2H2O+H2
2MnO4-+3H2O2→ 2MnO2+3O2+2H2O+2OH-
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Hydrogen peroxide is produced by acidifying barium peroxide and eliminating surplus water by evaporation under low pressure. Water is used to extract it, then distillation under lower pressure concentrates it to around 30% (by mass). Careful distillation under low pressure can increase the concentration to 85%. To achieve pure H2O2, the residual water can be frozen out.
Uses of H2O2:
(i) As an antiseptic it is sold in the market as perhydrol.
(ii) It is used to manufacture chemicals like sodium perborate and per - carbonate. It is employed in the industries as a bleachin
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
D2O can be prepared by prolonged electrolysis of water. Due to high molecular mass D2O differs from water.
NaOH+D2O → NaOD+HOD
HCl+D2O → DCl+ HOD
NH4Cl+D2O → NH3DCl+HOD
Physical Properties of H2O and D2O
Property | H2O | D2O |
Molecular mass (g mol-1) | 18.015 | 20.0276 |
Melting point/K | 273.0 | 276.8 |
Boiling point/K | 373.0 | 374.4 |
Enthalpy of formation/kJ mol-1 | -285.9 | -294.6 |
Enthalpy of Vaporisation (373 K)/kJ mol-1 | 40.66 | 41.61 |
Enthalpy of fusion/kJ mol-1 | 6.01 | — |
Temp of max. density/K | 276.98 | 284.2 |
Density (298 K)/g cm-3 | 1.0000 | 1.1059 |
Viscosity/centipoise | 0.8903 | 1.107 |
Dielectric constant | 78.39 | 78.06 |
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
New answer posted
8 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Atomic hydrogen is highly reactive, whereas molecular hydrogen is rather inert. Bond dissociation enthalpy determines the chemical behaviour of dihydrogen (and, for that matter, any molecule) to a great extent. For a single bond between two atoms of any element, the H-H bond dissociation enthalpy is the highest. As a result, molecular hydrogen reacts only with a few elements.
New answer posted
9 months agoContributor-Level 10
9.56. Dihydrogen is prepared from water by the action of alkali metals like Na and K which is a strong reducing agent.
2Na + 2H2O → 2NaOH + H2
2K + 2H2O → 2KOH + H2
New answer posted
9 months agoContributor-Level 10
9.54. Water which does not produce lathers with soap is known as hard water. Hardness is due to the presence of bicarbonates, sulphates and chlorides of Ca2+ and Mg2+.
On boiling, the bicarbonates of calcium and magnesium decompose to insoluble carbonate which can be removed by filtration.
New answer posted
9 months agoContributor-Level 10
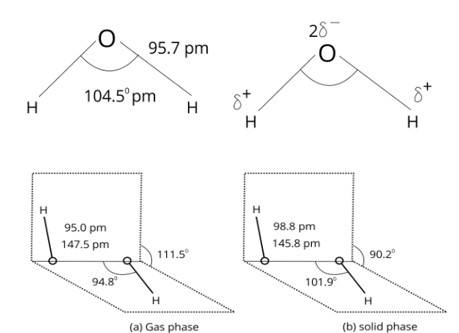
9.53. In water molecule, O is SP3 hybridized. Due to stronger lone pair-lone pair repulsion than bond pair-bond pair repulsions, the H-O-H bond angle decreases from 109.5° to 104.5°. Thus, water is bent molecule.
New answer posted
9 months agoContributor-Level 10
9.52. Many transition and inner-transition metals absorb hydrogen into the interstices of their lattices to yield metal like hydrides also called the interstitial hydrides. These hydrides are generally non stoichiometric and their composition vary with temperature and pressure.
For example, TiH1.73, CeH2.7
Two uses of interstitial hydrides are:
(i) In the storage of H2.
(ii) Catalyst for hydrogenation reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
