Kinetic Theory
Get insights from 102 questions on Kinetic Theory, answered by students, alumni, and experts. You may also ask and answer any question you like about Kinetic Theory
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Radius = 1Ao
Volume of hydrogen molecules = 4/3 r3
= 4/3 (3.14) (10-10)3 m3
Number of moles of H2 = mass/molecular mass=0.5/2=0.25
Molecules of H2 present = number of moles of H2 present
= 0.25
So volume of molecules present = molecule number volume of each molecules
= 0.25
6 3
PiVi= PfVf
Vf = i= 3
Vf= 2.7 3
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(a) The average KE will be the same
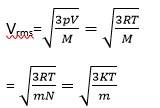
M= molar mass of the gas
m=mass of each molecular of the gas
R= gas constant
vrms
(b) k = Boltzmann constant
T= absolute temperature
mA>mB>mC
Vrms.A
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
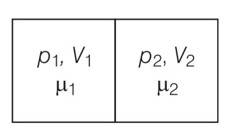
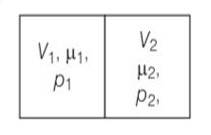
V1=2L ,V2=3L
µ1 = 4.0and µ2 =5.2
p1= 1.00 atm and p2 = 2.00 atm
p1V1= µ1RT1
p2V2= µ2RT2
when the partition is removed the gases get mixed without any loss of energy . the mixture now attains a common equilibrium pressure and total volume of the system is sum of the volume of individual chambers V1 and V2
, V =V1+V2
From the kinetic theory of gases pV=2/3 E
For mole 1 ,P1V1= 2/3
For mole 2 , P2V2= 2/3
Total energy is ( )= 3/2 ( )
PV==2/3Etotal = 2/3
P( )=
P= =
P=8/5 =1.6atm
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Mean free path l=1/
So n= number of molecules /volume
d = diameter of the molecule
l
d1=1Ao, d2=2Ao
l
l1:l2=4:1
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Oxygen gas having 5 degrees of freedom
Energy per mole of the gas =5/2RT
For 2 moles of the gas total internal energy =2 5/2RT=5RT
Neon is a monoatomic gas having 3 degrees of freedom
Energy per mole =3/2RT
We have 4 moles of Ne
Energy = 4 3/2RT=6RT
Total energy =5RT+6RT=11RT
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
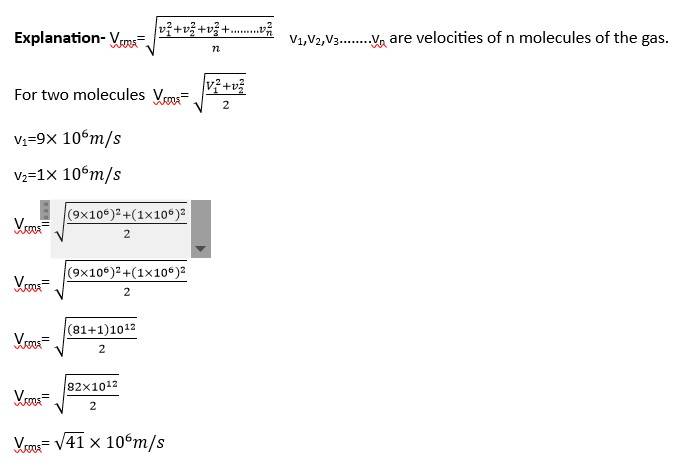
Vrms=
Vrms=
T1=27oC= 27+273=300K
T2=127oC= 127+273= 400K
Vrms2=
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
V
V/T = constant
T1=273+27=300K
T2= 273+327= 600K
V1= 100cc
V2=V1 (600/300)
V2=2V1
V2= 2 (100)=200cc
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
molar mass = mass of avogadro's number of atoms= 6.023 atoms.
197 g of gold contains =6.023
1g of gold contain= atoms
39.4 g of gold atoms = atoms
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers