Kinetic Theory
Get insights from 102 questions on Kinetic Theory, answered by students, alumni, and experts. You may also ask and answer any question you like about Kinetic Theory
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
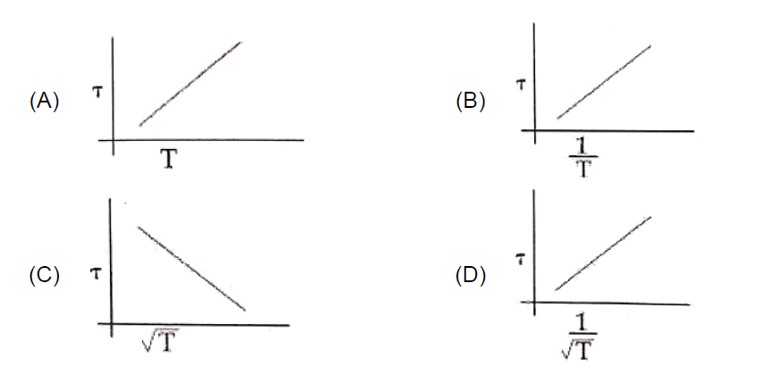
Frequency of collisions = 1/T = Vavg/λ = 600/ (3 * 10? ) = 2 * 10? sec? ¹
New answer posted
6 months agoContributor-Level 10
V_rms = √ (3RT/M)
V_N? = V_H?
√ (3RT_N? / M_N? ) = √ (3RT_H? / M_H? )
573/28 = T_H? /2
⇒ T_H? = 40.928
New answer posted
6 months agoContributor-Level 10
λ = kT / (√2πd²P)
= (1.38*10? ²³ * 300) / (√2 * 3.14 * (0.3*10? )² * 1.01*10? )
≈ 102 nm
New answer posted
6 months agoContributor-Level 10
1 litre, T = 300K, P = 2 atm, KE = 2*10? J/molecule, no of molecule =?
No. of molecules = (no of moles) * NA = nNA
Also, n = PV/RT = PV/ (NAkT)
KE = (3/2)kT = 2*10? J [Given]
kT = (4/3)*10?
P = 2 atm = 2 * 1.013 * 10? N/m²
vol = 1 lit = 10? ³ m³
No. of molecules = PV/kT = (2*1.013*10? * 10? ³)/ (4/3)*10? ) ≈ 1.5 * 10¹¹
New answer posted
6 months agoContributor-Level 10
According to KTG, the gas exerts pressure because its molecule :
suffer change in momentum when impinge on the walls of container.
New answer posted
6 months agoContributor-Level 10
Translational kinetic energy will be equal to rotational kinetic energy corresponds to each degree of freedom.
New answer posted
6 months agoContributor-Level 10
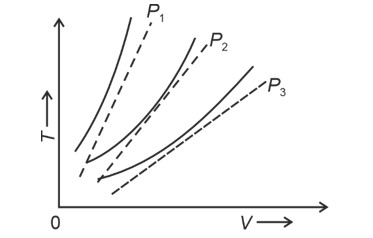
At same temperature, curve with higher volume corresponds to lower pressure.
(We draw a straight line parallel to volume axis to get this)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers